Comment:
This article shows a benefit to soy intake for ER+/PR+ patients taking AI medications, but not with tamoxifen.
Summary:
Clinical Bottom Line
This prospective cohort study found a significant association between high dietary soy isoflavone intake (defined as >42.3 mg/day) and a reduced risk of breast cancer recurrence among postmenopausal patients receiving adjuvant endocrine therapy. This potential benefit was most pronounced in patients with estrogen and progesterone receptor-positive (ER+/PR+) tumors and in those receiving anastrozole. Conversely, the study found no association between soy intake and risk of recurrence or death in premenopausal patients. As an observational study, these findings show an association and cannot prove that soy intake causes the reduced recurrence; the results may be influenced by unmeasured confounding variables. The findings are specific to a Chinese population with relatively high baseline soy consumption and may not be generalizable to populations with lower soy intake.
Results in Context
-
Main Results: The study followed 524 patients for a median of 5.1 years. Patients were categorized by daily soy isoflavone intake into quartiles.
-
Premenopausal Patients ($n=248$): There was no statistically significant association between soy isoflavone intake and breast cancer recurrence or death. Comparing the highest intake quartile (>$42.3 \text{ mg/day}$) to the lowest (<$15.2 \text{ mg/day}$), the adjusted hazard ratio (HR) for recurrence was 0.88 (95% CI 0.61–1.23) and for death was 1.05 (95% CI 0.78–1.71).
-
Postmenopausal Patients ($n=276$): A significant inverse association was seen for recurrence. Patients in the highest intake quartile had a significantly lower risk of recurrence compared to those in the lowest quartile (Adjusted HR 0.67, 95% CI 0.54–0.85; p for trend = 0.02). There was no significant association with all-cause mortality (Adjusted HR 0.88, 95% CI 0.56–1.24).
-
-
Subgroup Analyses (Postmenopausal): The beneficial association for recurrence was restricted to specific subgroups:
-
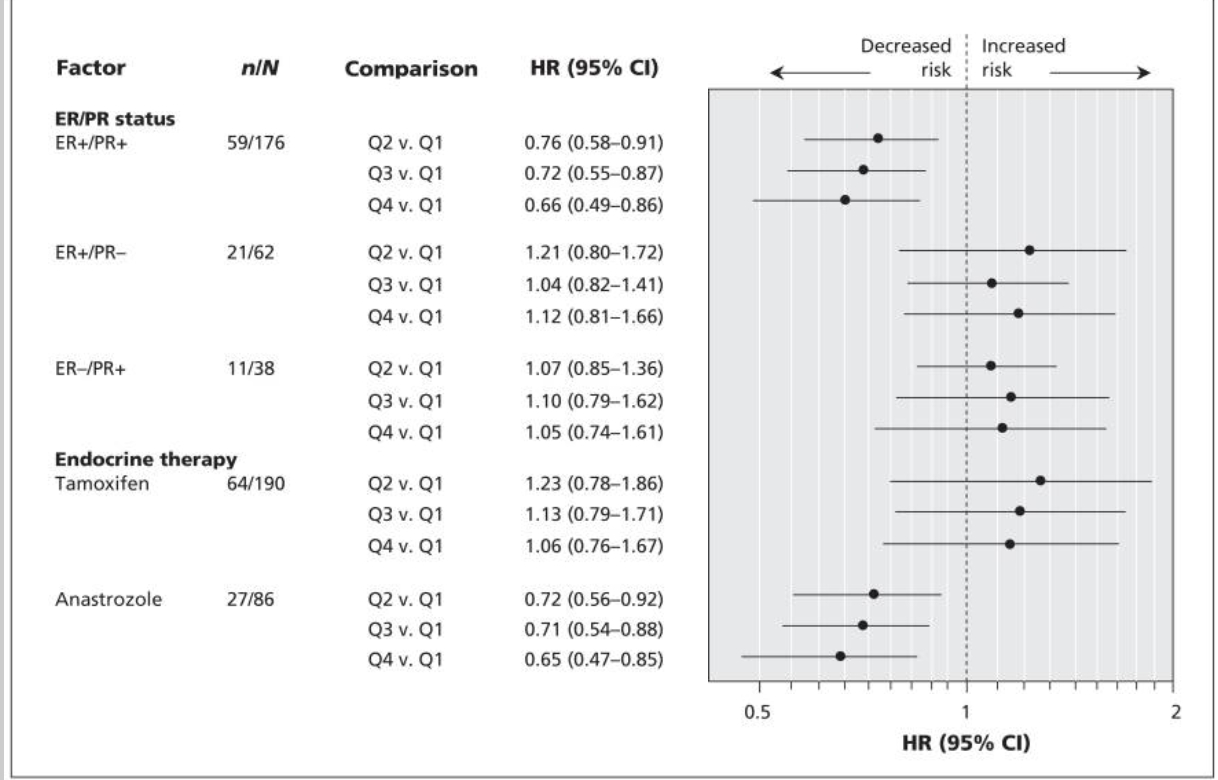
ER+/PR+ Tumors: Patients with ER+/PR+ disease in the highest soy intake quartile had a significantly lower risk of recurrence (HR 0.66, 95% CI 0.49–0.86).
-
Anastrozole Therapy: Patients receiving anastrozole in the highest soy intake quartile had a significantly lower risk of recurrence (HR 0.65, 95% CI 0.47–0.85).
-
Tamoxifen Therapy: No significant association was observed for patients receiving tamoxifen (HR 1.06, 95% CI 0.76–1.67).
-
-
Definitions: A hazard ratio (HR) represents the risk of an event (like recurrence) in one group compared to another over time. An HR of 0.67 means the high-intake group had a 33% lower hazard of recurrence compared to the low-intake group. The 95% confidence interval (CI) not crossing 1.0 indicates this finding is statistically significant.
-
Participants: The study included 524 women with early or advanced breast cancer from Harbin, China. All patients were receiving adjuvant endocrine therapy and had ER+, PR+, or ER+/PR+ tumors. Of these, 248 (47.3%) were premenopausal and 276 (52.7%) were postmenopausal.
Assertive Critical Appraisal
-
Limitations & Bias (STROBE Framework):
-
Confounding: As with all observational studies, the risk of unmeasured confounding is a primary limitation. While the authors adjusted for known risk factors (age, TNM stage, receptor status, chemotherapy, radiotherapy, and endocrine therapy), other unmeasured lifestyle or dietary factors could be responsible for the observed association. The authors also note that other bioactive components in soy, such as soy protein, could be influencing the results.
-
Recall Bias: Dietary intake was assessed at baseline using a food frequency questionnaire (FFQ) that asked participants to recall their usual consumption over the previous five years. This long recall period introduces a high potential for recall bias and inaccurate reporting of soy intake.
-
Generalizability: The study was conducted in a specific Chinese population with a mean daily isoflavone intake (25.6 mg/day) typical of Asian countries. The authors correctly caution that these findings may not be generalizable to other populations, such as Western populations, where soy consumption is substantially lower.
-
Measurement: The study relied entirely on self-reported dietary intake. No biological samples (e.g., blood or urine) were collected to objectively measure and verify isoflavone levels.
-
-
Reporting Quality Assessment (STROBE): The paper adheres to STROBE guidelines by clearly describing the efforts to address confounding, listing the variables adjusted for in the multivariable models. It also provides a clear description of participant enrollment and follow-up.
Applicability
The study’s findings are most relevant to postmenopausal breast cancer survivors with ER+/PR+ disease, particularly those taking anastrozole, who live in regions with high baseline soy consumption. The results challenge the long-standing concern that soy’s weak estrogenic effects might be harmful to patients with hormone-sensitive breast cancer. However, these data are not strong enough to recommend increasing soy intake to prevent recurrence, nor do they apply to premenopausal patients.
Research Objective
The study’s objective was to examine the associations between dietary intake of soy isoflavones and the recurrence of breast cancer and death for patients who had undergone surgery for breast cancer and were receiving adjuvant endocrine therapy.
Study Design
This was a prospective cohort study. Researchers recruited 524 women with breast cancer who were receiving adjuvant endocrine therapy. At baseline, dietary intake of soy isoflavones over the previous five years was assessed using a validated food frequency questionnaire. Participants were then followed prospectively for a median of 5.1 years to ascertain outcomes of cancer recurrence and death. Associations were analyzed using multivariable Cox proportional hazards regression models to adjust for known risk factors.
Setting and Participants
-
Setting: Patients were recruited from the Cancer Hospital of Harbin Medical University in Harbin, China.
-
Participants: The study included 524 women diagnosed with early or local advanced breast cancer who had undergone surgery and were receiving adjuvant endocrine therapy. All patients had tumors positive for estrogen receptor, progesterone receptor, or both.
-
Dates: Patients were recruited between August 2002 and July 2003 and were followed until July 2008.
Bibliographic Data
-
Title: Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy
-
Authors: Xinmei Kang, Qingyuan Zhang, Shuhuai Wang, Xu Huang, Shi Jin
-
Journal: CMAJ (Canadian Medical Association Journal)
-
Year: 2010
-
DOI: 10.1503/cmaj.091298
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text Pubmed Central
