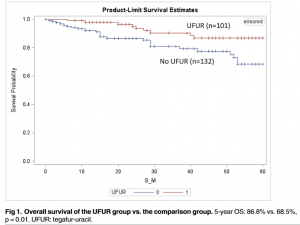
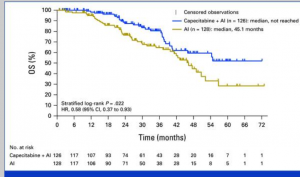
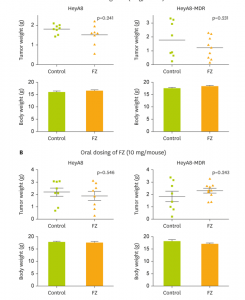
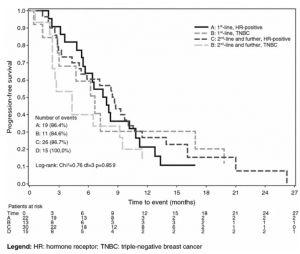
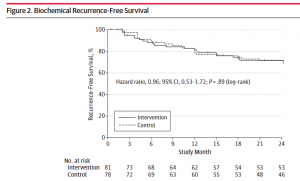
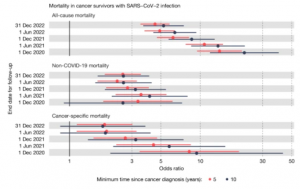
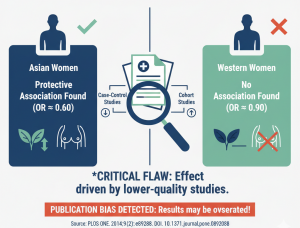
Reporting of Clinical Trial Uncertainties With New Cancer Drugs in Journal Publications and Clinical Guidelines

Comment: Unfortunately, this study confirms a persistent issue. We repeatedly see this major discrepancy between the “known unknowns”—the clinical uncertainties the FDA rightly identifies during expedited approvals—and what actually gets published in high-impact journals and translated into clinical guidelines like the NCCN. The result is that frontline oncologists are often making treatment decisions with an […]