Comment:
While this study is flawed by its retrospective design and critical selection bias, we shouldn’t discard the core hypothesis. The authors are right to call the findings preliminary, but the concept of metronomic maintenance therapy remains highly compelling.
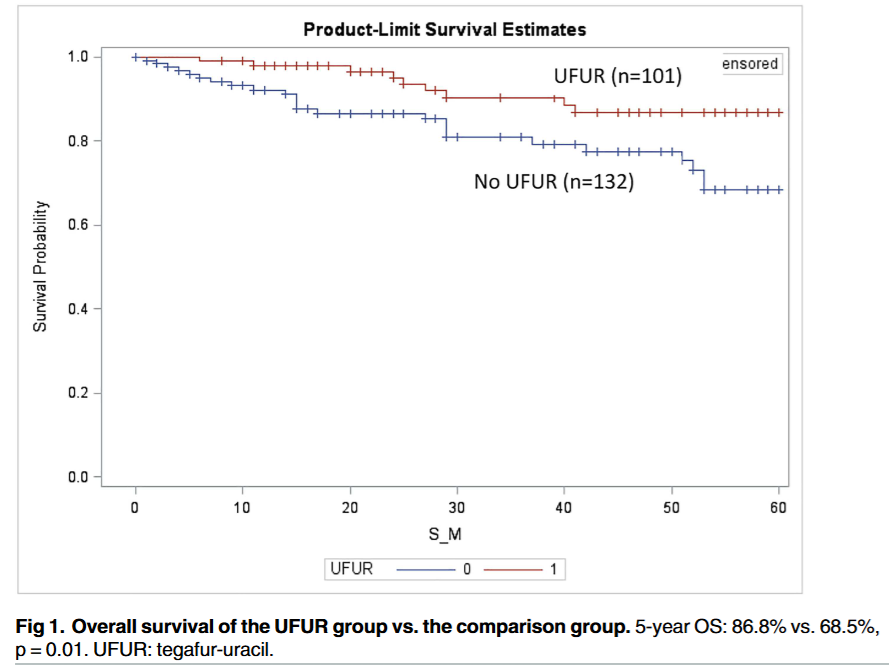
The fact that such a strong Overall Survival signal (86.8% vs. 68.5%) was detected at all, even within this biased framework, is intriguing. It highlights the potential for a well-tolerated, convenient, low-dose oral agent to improve long-term outcomes after the rigors of standard FOLFOX. While these specific results are unreliable, they provide a powerful, hypothesis-generating rationale for a properly designed, prospective randomized controlled trial to investigate this strategy.
Summary:
Clinical Bottom Line
This retrospective study shows a strong association between adding metronomic oral tegafur-uracil (UFUR) after standard adjuvant FOLFOX and improved 5-year overall survival (OS) for patients with stage III colon cancer (86.8% vs. 68.5%). However, this study cannot prove causation due to a critical flaw: selection bias. Physicians assigned UFUR based on their “personal preference”, meaning patients who received the maintenance therapy may have been healthier, more motivated, or had other unmeasured positive attributes. This bias, not the drug itself, could explain the survival difference. Notably, the study found no significant difference in disease-free survival (DFS). Therefore, these findings are highly preliminary and should not change clinical practice without confirmation from a randomized controlled trial.
Results in Context
Main Results
The study compared 113 patients who received FOLFOX followed by oral UFUR (UFUR group) with 132 patients who received FOLFOX alone (comparison group).
-
Overall Survival (OS): The primary positive finding was a statistically significant improvement in 5-year OS. The UFUR group had a 5-year OS rate of 86.8%, compared to 68.5% in the comparison group (p = 0.0107).
-
Disease-Free Survival (DFS): There was no statistically significant difference in 5-year DFS between the two groups. The 5-year DFS rate was 69.1% in the UFUR group versus 62.3% in the comparison group (p = 0.21).
-
Subgroup Analysis: A subgroup analysis based on treatment duration suggested the OS benefit was only seen in patients who received UFUR for ≥ 5 months (5-year OS 90.3%). Those taking it for <5 months had no survival benefit over the comparison group.
Definitions
-
Overall Survival (OS): Defined as the time from the day of diagnosis to the day of death from any cause.
-
Disease-Free Survival (DFS): Defined as the time from the diagnostic day to the day of locoregional recurrence or distant metastasis.
-
p-value: The study considered a p-value of 0.05 or less to be statistically significant. The p = 0.0107 for OS show statistical significance between the group due to the intervention.
Participants
A total of 245 patients were included from a single hospital’s database. All had stage III colon carcinoma, had undergone a radical R0 resection, and had received postoperative adjuvant FOLFOX chemotherapy. Patients with rectal cancer were excluded.
Assertive Critical Appraisal
Limitations & Bias (STROBE Framework)
The study’s conclusions are severely weakened by its methodology.
-
Critical Selection Bias: This is the study’s fatal flaw. It is a retrospective, non-randomized study. The authors explicitly state that “physicians prescribed the drug according to their personal preference”. This introduces a massive risk of confounding by indication (a type of selection bias). It is highly probable that physicians preferentially offered maintenance therapy to patients who were healthier, had a better performance status, or were more compliant. These unmeasured factors, rather than the UFUR itself, could easily account for the entire survival benefit.
-
Contradictory Endpoints: It is unusual to see a significant OS benefit with no corresponding benefit in DFS. The authors speculate this might be due to UFUR inhibiting metastasis, leading to a lower disease burden at relapse, but this is only a hypothesis. This discrepancy further raises questions about the validity of the OS finding.
-
Lack of Standardization: The duration of UFUR therapy was not standardized, ranging from 3 to 12 months. The subgroup analysis finding that ≥ 5 months of therapy was required for benefit is also subject to selection bias, as patients who are healthier are more likely to tolerate and continue therapy for a longer period.
Reporting Quality Assessment (STROBE)
The authors do not clearly describe their efforts to address potential sources of confounding, which is the most critical methodological challenge in this study. While baseline characteristics like stage and grade were reported as similar, the paper fails to perform any statistical adjustments (e.g., multivariate analysis or propensity score matching) to account for the non-random assignment. This omission means the comparison is not reliable, and the reported survival difference cannot be confidently attributed to the UFUR.
Reporting Quality Assessment (RECORD) for RWE Studies
This study uses real-world data from a hospital registry. The authors adequately describe the data source, how participants were selected (stage III colon cancer, R0 resection, post-op FOLFOX), and how outcomes were defined. The critical weakness remains the non-randomized allocation of the intervention (UFUR).
Applicability
The intervention, oral metronomic UFUR, is convenient and reported as well-tolerated (though it caused significantly more anorexia). However, given the major methodological flaws, especially the high risk of selection bias, these results are not robust enough to support changing clinical practice. The study’s findings are, at best, hypothesis-generating and would require validation in a large, prospective, randomized controlled trial.
Research Objective
The study’s purpose was to estimate the impact of metronomic oral tegafur-uracil (UFUR) following a standard intravenous FOLFOX regimen on the overall survival (OS) and disease-free survival (DFS) of patients with stage III colon cancer.
Study Design
This was a retrospective cohort study conducted at a single institution. Patient data was reviewed from a hospital database from October 2008 through December 2014. The study compared a cohort of 113 patients who received adjuvant FOLFOX followed by metronomic UFUR with a cohort of 132 patients who received adjuvant FOLFOX alone.
Setting and Participants
-
Setting: Tri-Service General Hospital in Taipei, Taiwan.
-
Participants: 245 patients with newly diagnosed stage III colon carcinoma who had a radical R0 resection and received postoperative adjuvant FOLFOX.
-
Key Exclusions: Patients with rectal cancer were excluded.
Bibliographic Data
-
Title: Oral tegafur-uracil as metronomic therapy following intravenous FOLFOX for stage III colon cancer.
-
Authors: Huang W-Y, Ho C-L, Lee C-C, Hsiao C-W, Wu C-C, Jao S-W, Yang J-F, Lo C-H, Chen J-H.
-
Journal: PLoS ONE.
-
Year: 2017.
-
DOI: 10.1371/journal.pone.0174280
Mandatory Disclaimer: This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical cond1ition.
Original Article:
Full text pdf: Oral tegafur-uracil as metronomic therapy following intravenous FOLFOX for stage III colon cancer
Copyright: © 2017 Huang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
