Comment:
Two underlying assumptions of the repurposed drug movement is that since the medications are already approved they are both safe and can achieve clinically meaningful levels. The issue is that using an anti-parasitic short term is very different than trying to get higher blood levels long term.
This trial shows delayed, grade 3 liver enzyme elevation in a subset of patients at the highest dose—establishing a clear safety concern.
However, the most significant finding is that even at this toxicity-limiting dose, the plasma concentrations remained substantially lower (approximately four times) than the levels associated with anti-cancer efficacy in preclinical models bringing into question whether a clinical efficacious does is even possible to achieve.
Summary:
Clinical Bottom Line
This Phase 1 dose-escalation study found that high-dose mebendazole (up to 200 mg/kg/day) added to standard adjuvant temozolomide has an acceptable safety profile for patients with newly diagnosed high-grade gliomas. The primary risk identified was delayed, reversible grade 3 liver enzyme (ALT/AST) elevation, which occurred in 4 of 15 patients at the highest dose but resolved with dose reduction or discontinuation.
However, the study highlights a significant challenge: plasma levels achieved in patients were highly variable and remained substantially lower than the concentrations associated with anti-cancer efficacy in preclinical models. Even at the highest dose, mean trough plasma concentrations were approximately four times lower than the 1,000 ng/mL level deemed effective in mouse models, suggesting that achieving a therapeutically relevant dose with this formulation may be difficult.
Results in Context
Main Results: Safety and Potential Risks
The study’s primary goal was to find the maximum tolerated dose (MTD) and assess safety.
-
Dose-Limiting Toxicity (DLT): No DLTs (defined as grade $\ge$ 3 non-hematologic or grade $\ge$ 4 hematologic toxicity in the first 28-day cycle) were observed, even at the highest dose of 200 mg/kg/day. This led to dose escalation to the highest planned level.
-
Delayed Toxicity: The main risk appeared after the first month. Four of 15 patients (16.7%) in the 200 mg/kg/day cohort developed delayed grade 3 elevations in ALT and/or AST between months 2 and 5.
-
Reversibility: These liver enzyme elevations were reversible in all cases, either by lowering the mebendazole dose to 100 mg/kg or by discontinuing the drug. No severe adverse events (requiring hospitalization or causing death) were attributed to mebendazole.
Main Results: Pharmacokinetics and Dosing Levels
A secondary goal was to measure plasma levels.
-
Plasma Concentrations: Plasma levels of mebendazole were highly variable among patients.
-
Sub-Therapeutic Levels: At the highest dose (200 mg/kg/day), the mean steady-state trough plasma concentration (Cmin) was 261.0 $\pm$ 126.4 ng/mL.
-
Context: The authors note that in mouse models, plasma levels in excess of 1,000 ng/mL were observed with effective doses. The study concludes that “Further studies would be required to see if these levels could be safely achieved in humans”.
-
Dose-Normalization: The study also found a statistically significant decrease in dose-normalized exposure at the 200 mg/kg/day level, suggesting that doubling the dose from 100 mg/kg did not double the plasma concentration.
Participants
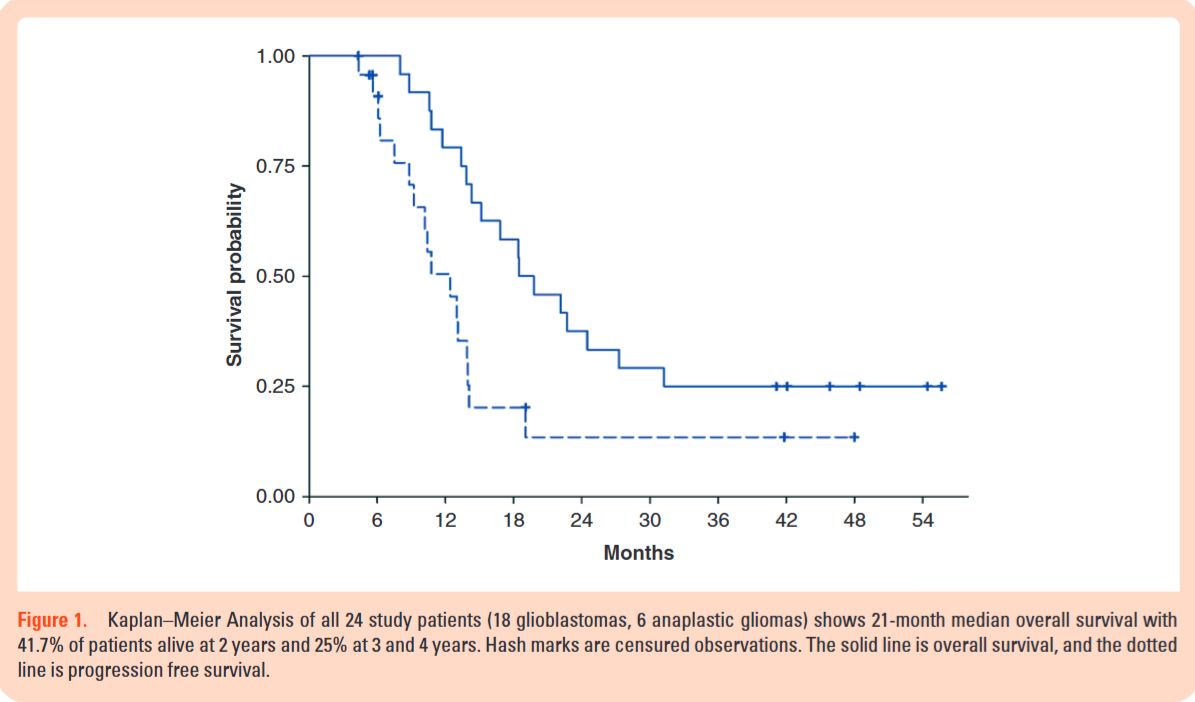
A total of 24 patients with newly diagnosed high-grade gliomas were enrolled (18 glioblastoma, 6 anaplastic glioma). All patients had completed standard postoperative chemoradiation with temozolomide before starting the trial.
Assertive Critical Appraisal
Limitations & Bias
As a single-arm, non-randomized Phase 1 trial, this study’s primary and only reliable conclusions are related to safety and pharmacokinetics.
-
Small Sample Size: With only 24 patients total, and only 15 at the highest dose, the ability to detect rare adverse events is limited.
-
No Causal Inference: The study’s survival data (21-month median overall survival) is preliminary and hypothesis-generating at best. The authors correctly note that this small, heterogeneous (Grades III and IV) cohort and potential for sample bias mean the survival finding “may be difficult to reproduce”. This study cannot provide any evidence that mebendazole is effective.
Reporting Quality Assessment
The study adheres to the principles of STROBE by clearly defining its objectives (safety and MTD), population, and outcomes. Crucially, the authors are transparent about the study’s limitations and appropriately avoid overstating the preliminary survival findings. They also explicitly warn against off-label use, citing the potential for side effects (like liver toxicity) and differences in drug bioavailability.
Applicability
For clinicians, this study confirms that high-dose mebendazole can be administered with temozolomide, but it requires diligent safety monitoring, specifically monthly blood counts and serum chemistry, to watch for liver toxicity.
The study’s pharmacokinetic data is a major caution. The failure to reach plasma levels deemed therapeutic in animals suggests that the anti-cancer activity of this specific mebendazole formulation (polymorph C) may be limited by its bioavailability. The authors suggest that pill burden and PK data might make a 75-100 mg/kg/day dose or a “formulation with greater bioavailability” preferable for future efficacy trials.
Research Objective
The primary objectives were to determine the maximum tolerated dose (MTD) of mebendazole when given in combination with standard adjuvant temozolomide and to evaluate the safety and toxicity of this combination in patients with newly diagnosed high-grade gliomas. Secondary objectives included estimating plasma concentrations and obtaining preliminary estimates of overall and progression-free survival.
Study Design
This was a single-institution, open-label, nonrandomized phase 1 trial. It used a standard 3+3 dose-escalation design to test mebendazole doses of 25, 50, 100, and 200 mg/kg/day. An expansion cohort of 15 patients was planned at the MTD or highest dose, which resulted in 15 patients being enrolled at the 200 mg/kg/day level.
Setting and Participants
The study was conducted at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University. Participants were 24 adult patients (≥18 years) with histologically confirmed, newly diagnosed malignant glioma (WHO Grade III or IV) who had already completed standard concurrent radiation and temozolomide.
Bibliographic Data
-
Title: Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: results of a phase 1 clinical trial
-
Authors: Gary L. Gallia, Matthias Holdhoff, Henry Brem, Avadhut D. Joshi, Christine L. Hann, Ren-Yuan Bai, Verena Staedtke, Jaishri O. Blakeley, Soma Sengupta, T. Che Jarrell, Jessica Wollett, Kelly Szajna, Nicole Helie, Austin K. Mattox, Xiaobu Ye, Michelle A. Rudek, and Gregory J. Riggins
-
Journal: Neuro-Oncology Advances
-
Year: 2021
-
DOI: 10.1093/noajnl/vdaa154
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text pdf: Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas results of a phase 1 clinical trial
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
