Comment:
This study presents a very practical and appealing option for managing advanced HER2-negative breast cancer in the palliative setting. The key takeaway is the dual advantage of this all-oral metronomic regimen: it demonstrates clinically relevant activity while maintaining an exceptionally low toxicity profile.
However, the regimen’s main strength is its safety and convenience. Being all-oral, it frees patients from hospital visits for IV infusions. More importantly, the toxicity is remarkably low. The complete absence of severe alopecia is a significant quality-of-life benefit that cannot be overstated. Furthermore, the per-cycle rate of severe (Grade 3-4) toxicities like neutropenia or hand-foot syndrome was only about 1%.
While this non-randomized study doesn’t prove superiority, it strongly supports this combination as a compelling option for patients who prioritize quality of life, wish to avoid harsh side effects, or may not be candidates for more aggressive IV chemotherapy. It’s an excellent example of how metronomic therapy can optimize disease control with minimal patient burden.
Summary:
Clinical Bottom Line
This phase II, single-arm study suggests that the all-oral metronomic (low-dose, continuous) combination of vinorelbine and capecitabine is a well-tolerated and active regimen for patients with advanced HER2-negative breast cancer. In 80 evaluable patients, the combination demonstrated promising activity, particularly in the second-line setting with a Clinical Benefit Rate (CBR) of 51.1%. The regimen’s primary advantage is its excellent safety profile, with a very low rate of severe side effects (like neutropenia or hand-foot syndrome) and a lack of severe alopecia.
As a non-randomized, open-label study that was stopped early, it cannot prove this regimen is better than standard care. However, its convenience and high tolerability make it a compelling palliative option for controlling disease, especially for patients who wish to avoid hospital visits or more toxic IV chemotherapy.
Results in Context
Main Results
The study’s primary endpoint was the Clinical Benefit Rate (CBR), defined as the proportion of patients achieving a complete response, partial response, or stable disease lasting at least 24 weeks.
-
Overall Population (N=80): The CBR was 48.8% (95% CI 37.4-60.2).
-
By Treatment Line:
-
First-line (N=35): CBR was 45.7% (95% CI 28.8-63.4).
-
Second-line (N=45): CBR was 51.1% (95% CI 35.8-66.3).
-
-
By Tumor Subtype (Overall):
-
HR-positive (N=52): CBR was 55.8% (95% CI 41.3-69.5).
-
Triple-Negative (TNBC, N=28): CBR was 35.7% (95% CI 18.6-55.9), which the authors noted as clinically relevant.
-
-
By Visceral Involvement (Overall):
-
With visceral involvement: 44.8% (95% CI 31.7-58.5)
-
Without visceral involvement: 59.1% (95% CI 36.4-79.3)
-
Secondary Outcomes
-
Objective Response Rate (ORR): The ORR (rate of tumor shrinkage) was 35.5% in the first-line group and 25.6% in the second-line group.
-
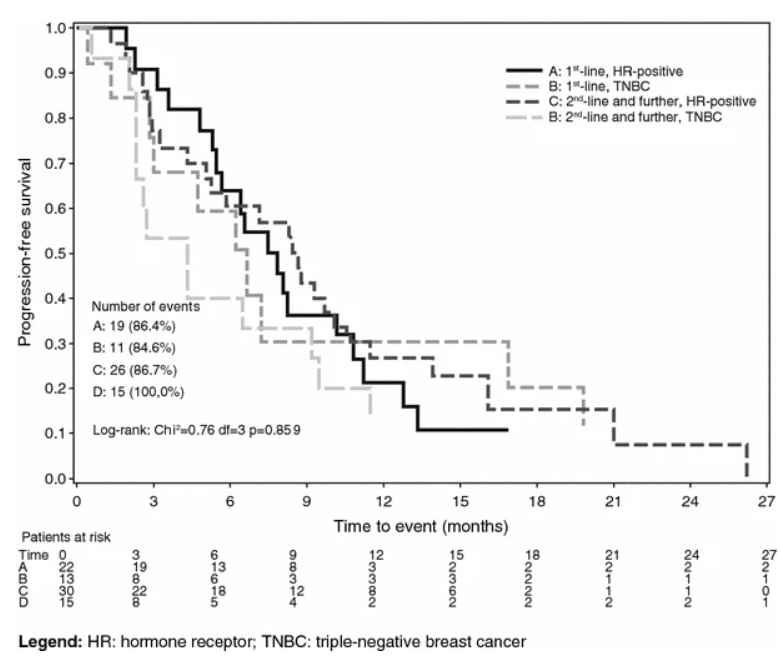
Progression-Free Survival (PFS): The median PFS was 6.7 months for the first-line group and 7.2 months for the second-line group. When analyzed by tumor biology, median PFS was 8.2 months in HR-positive patients versus 4.7 months in TNBC patients.
-
Harms and Adverse Events: The treatment was exceptionally well-tolerated.
-
Per-Patient (N=80): The most common severe (Grade 3-4) toxicities were nausea/vomiting (10.0%), hand-foot syndrome (10.0%), and leucopenia (8.8%). Febrile neutropenia was reported in 4 patients (5.0%).
-
Per-Cycle (N=896): The rate of severe toxicities per cycle was very low. The most frequent were non-febrile neutropenia (1.1% of cycles), hand-foot syndrome (1.0%), and nausea/vomiting (1.0%).
-
-
Notably, no severe (Grade 3-4) alopecia was observed.
Assertive Critical Appraisal
Limitations & Bias (STROBE Framework)
The study’s conclusions are significantly limited by its design and execution:
-
Single-Arm, Non-Randomized Design: This is the most critical flaw. As an open-label, phase II study, there was no control group. It is impossible to know if the observed outcomes (e.g., 7.2-month median PFS in the second-line) are any better or worse than standard-of-care chemotherapy. The results show the drug combination is active, but not its relative efficacy.
-
Premature Study Closure: The trial was stopped early due to slow recruitment in the first-line group.
-
Underpowered First-Line Analysis: The first-line group (Group 1) enrolled only 35 of the 61 patients required by the original statistical plan. The authors correctly state that this left the analysis with low power (~65%) and makes the first-line results “merely descriptive” and inconclusive. The second-line group, however, did meet its enrollment target.
-
Surrogate Endpoint: The primary endpoint was CBR, which includes stable disease. This is a common and appropriate endpoint for a phase II trial but is a weaker surrogate for patient benefit than PFS or the gold-standard Overall Survival.
Reporting Quality Assessment (STROBE)
The study’s reporting is generally transparent. The authors included a participant flow diagram (Fig. 1), which clearly shows that 86 patients were enrolled, 6 were excluded (4 screening failures, 2 missing data), and 80 were included in the primary analysis. The authors are also commendable for explicitly stating the limitations of their underpowered first-line cohort.
Applicability
The study’s findings are highly relevant for daily practice, particularly in the palliative setting. The population (HER2-negative advanced breast cancer) is common. The intervention’s main advantage is its “easy schedule of administration” as an all-oral regimen (mVNR 40 mg 3x/week, mCAPE 500 mg 3x/day). This, combined with its very low toxicity and lack of severe hair loss, makes it an attractive option for patients who prioritize quality of life, wish to minimize hospital visits, or may not be fit for more aggressive IV chemotherapy.
Research Objective
The VICTOR-2 study was an open-label, phase II trial designed to confirm the clinical activity and safety of an all-oral metronomic combination of vinorelbine and capecitabine in patients with advanced HER2-negative breast cancer. The primary objective was to evaluate the Clinical Benefit Rate (CBR).
Study Design
This was a prospective, single-arm, multicenter, open-label, phase II trial. All enrolled patients received the investigational treatment, which consisted of oral vinorelbine 40 mg (on Monday, Wednesday, Friday) and oral capecitabine 500 mg three times daily, administered continuously without drug-free periods. Patients were prospectively divided into two groups for analysis: Group 1 (first-line therapy) and Group 2 (second-line therapy).
Setting and Participants
-
Setting: The trial was conducted at 12 Italian centers.
-
Participants: 86 patients were enrolled between August 2011 and May 2015. 80 patients were evaluable for the primary efficacy analysis (35 in Group 1, 45 in Group 2).
-
Inclusion Criteria: Eligible patients were women (≥ 18 years) with documented locally advanced or metastatic HER2-negative breast cancer. Patients could be either chemotherapy-naïve (first-line) or previously treated (second-line).
-
Key Patient Characteristics (N=80):
-
Median Age: 65.3 years.
-
Receptor Status: 65% were HR-positive, 35% were TNBC.
-
Disease Burden: 72.6% had visceral disease, and 76.9% had >2 metastatic sites.
-
Bibliographic Data
-
Title: Metronomic chemotherapy with oral vinorelbine (mVNR) and capecitabine (mCAPE) in advanced HER2-negative breast cancer patients: is it a way to optimize disease control? Final results of the VICTOR-2 stud1y
-
Authors: M. E. Cazzaniga, L. Cortesi, A. Ferzi, et al., On behalf of VICTOR Study Group
-
Journal: Breast Cancer Research and Treatment
-
Year: 2016
-
DOI: 10.1007/s10549-016-4009-3
Original Article:
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
