Comment:
This study showed that supplementing with a soy protein isolate didn’t impact PSA levels.
However, a critical piece of context for this null finding is the dose they used. The 41 mg/day of total isoflavones used in this trial sits at the absolute low end of the 40-80 mg/day range typically tested in prostate cancer intervention studies.
This intervention is therefore more representative of a “high-dietary” intake rather than a true “pharmacological” supplement dose. The study’s conclusion is precise: this specific, high-dietary-level dose is ineffective for this high-risk population. This leaves the question open as to whether a higher pharmacological dose (e.g., 80 mg/day) would have yielded a different result.
And maybe next time without the sugar and strawberry flavoring 😉
Summary:
Clinical Bottom Line
This randomized, double-blind trial found that daily supplementation with 20 g of soy protein isolate for 2 years did not reduce or delay the biochemical recurrence of prostate cancer in men at high risk of recurrence following radical prostatectomy. The recurrence rates were nearly identical in the soy (27.2%) and placebo (29.5%) groups. The trial was appropriately stopped early at a planned interim analysis due to this clear lack of effect (futility). The intervention was safe and well-tolerated, but these findings do not support recommending soy protein supplements to this patient population for this indication.
Results in Context
Primary Outcome
The primary endpoints were the 2-year rate of biochemical recurrence and the time to recurrence. Recurrence was defined as a serum prostate-specific antigen (PSA) level of ≥ 0.07 n g / m L, confirmed by subsequent measurements.
The trial found no statistically significant difference between the groups:
-
Recurrence Rate: Recurrence occurred in 27.2% (22 of 81) of participants in the soy protein group and 29.5% (23 of 78) in the placebo group.
-
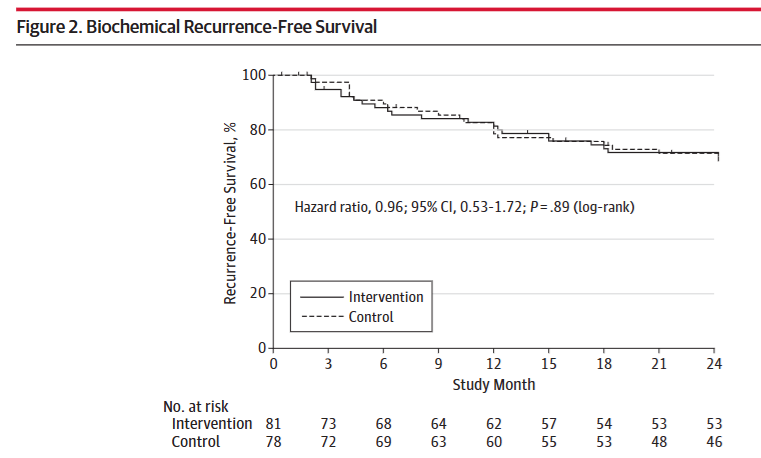
Hazard Ratio (HR): The HR for recurrence in the soy group was 0.96 (95% CI, 0.53-1.72; P = .89).
-
Interpretation: A hazard ratio of 0.96 means the rate of recurrence was virtually identical in both groups. The P value of .89 and the 95% confidence interval, which broadly overlaps 1.0, confirm this is a null finding.
Key Secondary & Specialized Outcomes
-
Oncology Endpoints & Response Criteria: The primary endpoint, biochemical recurrence (or “PSA failure”), is a surrogate endpoint. It is not a direct measure of clinical benefit, such as metastasis-free survival or overall survival (OS). The authors used this endpoint to identify effects on biologically significant cancers, but an effect on a surrogate does not always translate to patients living longer.
-
Patient-Reported Outcomes (PROs): The study did not formally report on PROs like quality of life.
Harms and Adverse Events
The intervention was found to be safe and well-tolerated.
-
There were no significant differences in the number or type of adverse events between the soy and placebo groups (see Table 3).
-
Gastrointestinal issues (e.g., constipation, bloating) were the most common potentially treatment-related complaints but occurred at similar low rates in both groups (7.4% in soy vs. 7.7% in placebo).
-
No deaths occurred during the study.
Assertive Critical Appraisal
Risk of Bias (RoB 2 Framework)
The overall risk of bias for this trial is low.
-
Randomization & Blinding: The study used a robust, stratified randomization procedure and was double-blind. All investigators, staff, and participants remained blinded.
-
Intervention & Placebo: The placebo (calcium caseinate) was packaged identically and flavored to mask taste differences, making blinding effective.
-
Missing Data: Of 177 randomized participants, 18 (10.2%) were excluded from the final analysis because they had no post-randomization PSA measurements (e.g., withdrew, ineligible baseline PSA). This created a “modified” intention-to-treat analysis, which is a minor limitation, but the exclusions appear justified and balanced. Dropout from the study after starting was low (8.2%) and similar between groups.
Reporting Quality Assessment (CONSORT)
The trial’s reporting quality is high.
-
Participant Flow: The paper includes a clear CONSORT flow diagram (Figure 1) that transparently accounts for all patients from screening through analysis.
-
Methods: The methods for randomization, allocation concealment, and blinding are all clearly described.
-
Adherence: Adherence was rigorously assessed using both self-report (daily calendars) and objective biochemical measurement (serum isoflavone levels). Adherence was excellent (> 90% self-reported). An analysis excluding nonadherent participants confirmed the null finding.
-
Stopping Early: A major strength is the paper’s transparency regarding the decision to stop the trial early for futility, based on a planned interim analysis and an extremely low conditional power of 0.0012.
Applicability
The study’s findings are directly applicable to a specific, high-risk population.
-
Patient Population: The results apply to men who have already had a radical prostatectomy and are at high risk of recurrence (e.g., positive margins, high Gleason score, extracapsular extension).
-
Generalizability: The authors correctly note that these findings cannot be generalized to men at average risk, for primary prostate cancer prevention, or for men with a different disease state (like active surveillance). The population was also 88-90% white, limiting generalizability to other racial groups.
-
Intervention: The intervention was a 20 g daily serving of soy protein isolate, which was standardized to contain specific amounts of isoflavones (the putative active constituents), including genistein and daidzein.
-
per 1 g of protein… 2.13 mg as aglycone equivalents, amounting to (in aglycone equivalents) 1.24 mg of genistein, 0.78 mg of daidzein, and 0.11 mg of glycitein.
-
total amount per 47-g daily serving:
Total isoflavones: 41 mg
Genistein: 24 mg
These results apply only to this specific formulation and dose, not necessarily to whole soy foods or different supplements. -
Research Objective
To determine whether daily consumption of a 20 g soy protein isolate supplement for 2 years reduces the rate of, or delays, biochemical recurrence of prostate cancer in men at high risk of recurrence after radical prostatectomy.
Study Design
-
Design: A randomized, double-blind, placebo-controlled, parallel-group trial.
-
Allocation: 1:1 randomization.
-
Participants: 177 eligible men were randomized.
-
Intervention Group (n=87): Received a daily beverage powder containing ~20 g of soy protein isolate.
-
Placebo Group (n=90): Received a daily beverage powder containing ~20 g of calcium caseinate.
-
Analysis: The primary “modified intention-to-treat” analysis included 81 participants in the intervention group and 78 in the placebo group who had at least one follow-up PSA measurement.
Setting and Participants
-
Setting: The trial was conducted at 7 US medical centers, although 95% of participants were enrolled from two sites in New York City (New York University School of Medicine and the Manhattan VA Medical Center).
-
Key Eligibility Criteria:
-
Had undergone radical prostatectomy for prostate cancer within the previous 4 months.
-
Had a postsurgical PSA level of < 0.07 n g / m L.
-
Met one or more criteria for high risk of recurrence, such as preoperative PSA > 20 n g / m L, final Gleason score ≥ 8, positive surgical margins, or extracapsular extension.
-
-
Key Exclusion Criteria: Included significant baseline intake of soy (more than once per week).
Bibliographic Data
-
Title: Effect of Soy Protein Isolate Supplementation on Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy: A Randomized Trial
-
Authors: Maarten C. Bosland, DVSc, PhD; Ikuko Kato, MD, PhD; Anne Zeleniuch-Jacquotte, MS, MD; et al.
-
Journal: JAMA
-
Year: 2013
-
DOI: 10.1001/jama.2013.7842
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text : PubMed Central
