Comment:
The MECCA trial, while certainly not practice-changing, provides an important signal for the potential of metronomic chemotherapy.
The key takeaway is the therapeutic window. Achieving a 9-month PFS benefit and a positive OS trend with a regimen that produced Grade 3 adverse events in only 15.1% of patients is compelling. This highlights the central promise of metronomic dosing: improving outcomes without the severe toxicity profile of traditional chemotherapy. While this specific regimen isn’t a new standard, the data strongly support the value of further investigating low-toxicity, long-term metronomic strategies. Whether adding metronomic chemotherapy to current first-line standard of a CDK4/6 inhibitor plus an AI will give similarly positive results remains to be seen.
Summary:
Clinical Bottom Line
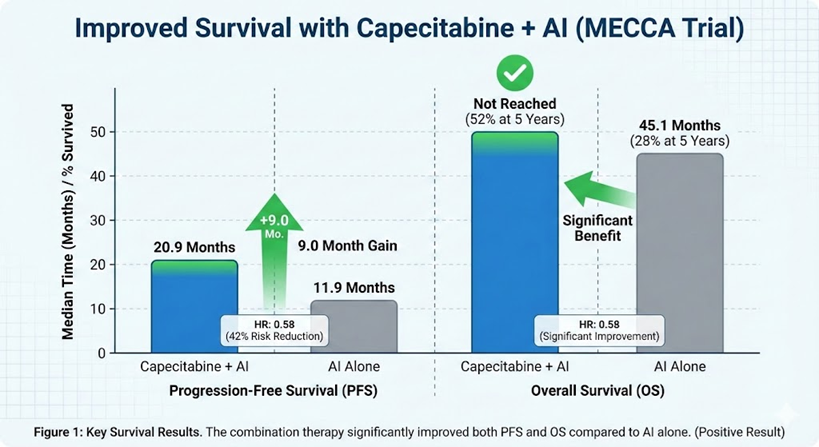
This phase III trial demonstrates that adding metronomic (low-dose, continuous) capecitabine to an aromatase inhibitor (AI) significantly improves both Progression-Free Survival (by 9.0 months) and Overall Survival compared to AI alone as first-line therapy for HR+/HER2- metastatic breast cancer.
However, this study must be viewed in its proper clinical context: the control arm (AI alone) is no longer the standard of care. The median PFS of 20.9 months in the combination arm, while a clear improvement, is still shorter than the 24–30 months typically seen with the current first-line standard (a CDK4/6 inhibitor plus an AI). Therefore, this regimen is not a new standard but rather a potential option for patients who are ineligible for, cannot tolerate, or do not have access to CDK4/6 inhibitors.
Results in Context
Primary Outcome
The primary endpoint was Progression-Free Survival (PFS).
-
The median PFS was 20.9 months in the capecitabine + AI arm.
-
The median PFS was 11.9 months in the AI alone arm.
-
The hazard ratio (HR) was 0.58 (95% CI, 0.43 to 0.76). This means there was a 42% reduction in the hazard (risk) of disease progression or death in the combination group compared to the AI alone group.
Key Secondary & Specialized Outcomes
-
Oncology Endpoints & Response Criteria:
-
Progression-Free Survival (PFS): The primary endpoint was a surrogate endpoint, measuring the time until cancer growth or death. While the 9-month improvement is clinically meaningful, surrogate endpoints do not always guarantee patients will live longer.
-
Overall Survival (OS): This trial did show a significant benefit in OS, the gold standard for clinical benefit. The median OS was not reached in the capecitabine + AI arm versus 45.1 months in the AI alone arm (HR, 0.58 [95% CI, 0.37 to 0.93]). The 5-year OS rate was 52% in the combination arm versus 28% in the AI arm.
-
Objective Response Rate (ORR): Measured using RECIST 1.1 criteria, the ORR was significantly higher in the combination arm (37.3%) compared to the AI alone arm (25.0%).
-
Disease Control Rate (DCR): Defined as disease controlled for 24 weeks or more, the DCR was also improved: 88.1% with the combination versus 75.8% with AI alone.
-
-
Patient-Reported Outcomes (PROs): PROs were listed as a secondary endpoint but were not reported in this publication.
-
Harms and Adverse Events:
-
The combination was generally tolerable. Grade 3 adverse events (AEs) occurred in 15.1% of patients in the combination arm.
-
The most common AE was palmar-plantar erythrodysesthesia (hand-foot syndrome), seen in 29.4% of the combination group (11.1% were Grade 3).
-
Other AEs more frequent in the combination arm included peripheral sensory neuropathy (8.7% vs 1.6%) and decreased neutrophil count (4.0% vs 0%).
-
More patients discontinued treatment due to AEs in the combination arm (16.7%) than in the AI alone arm (5.5%).
-
Assertive Critical Appraisal
-
Risk of Bias (RoB 2 Framework): The overall risk of bias is Some concerns.
-
The trial was open-label (not blinded), which creates a significant risk of bias. Since the primary endpoint (PFS) was investigator-assessed, knowledge of the treatment arm could have influenced the timing of progression assessment, potentially favoring the combination arm.
-
The randomization process used “the envelope method”. This is an outdated technique that is highly vulnerable to failure of allocation concealment (i.e., “peeking”) compared to a modern, centralized, computer-based system.
-
-
Subgroup Analyses: The subgroup analysis (Figure 4) showed a consistent benefit across most groups. However, these analyses are exploratory and should be interpreted with caution. The authors note a nearly significant interaction ( P = .054 ) for prior hormonal therapy, but this is not statistically significant and should be considered hypothesis-generating at best.
-
Appraisal of Patient-Reported Outcomes (CONSORT-PRO): The study protocol included patient-reported outcomes (PROs) as a secondary endpoint. However, this publication fails to report any PRO data. This is a critical omission, as it prevents any assessment of the combination’s impact on patient quality of life, especially given the known toxicities of capecitabine like hand-foot syndrome.
-
Reporting Quality Assessment (CONSORT): The paper’s reporting is generally good. It includes a CONSORT flow diagram (Figure 1), which clearly shows the flow of participants from screening to analysis. It also describes the randomization method (though the method itself is weak) and stratification factors.
-
Applicability: The key issue for applicability is the outdated control arm. AI monotherapy is no longer the first-line standard of care for most patients with HR+/HER2- MBC. The current standard, a CDK4/6 inhibitor plus an AI, provides a median PFS of approximately 24-30 months. The 20.9-month median PFS in the MECCA combination arm is clearly superior to AI alone but still inferior to the current standard. As the authors themselves suggest, the regimen’s applicability is likely limited to patients who are ineligible for, cannot tolerate, or do not have access to CDK4/6 inhibitors.
Research Objective
The study’s objective was to determine if adding metronomic capecitabine to an aromatase inhibitor (AI) improves progression-free survival (PFS) compared to AI alone in patients with hormone receptor-positive, HER2-negative metastatic breast cancer (MBC) who had not received prior systemic therapy in the metastatic setting.
Study Design
This was a randomized, controlled, open-label, phase III trial conducted at 12 centers in China. A total of 263 patients were randomly assigned in a 1:1 ratio. The efficacy analysis included 254 patients (126 in the combination arm, 128 in the AI al1one arm).
-
Experimental Arm: Metronomic capecitabine (500 mg three times a day, continuously) plus an AI (physician’s choice of anastrozole, letrozole, or exemestane).
-
Control Arm: AI alone.
-
Premenopausal or perimenopausal women in both arms also received a gonadotropin-releasing hormone agonist.
Setting and Participants
-
Setting: 12 medical centers in China.
-
Participants: Eligible patients were women aged 18-70 with hormone receptor-positive, HER2-negative metastatic breast cancer.
-
Key Inclusion Criteria: Patients must not have received any previous systemic therapy for their metastatic disease. Patients with measurable disease or nonmeasurable bone-only disease were eligible.
-
Key Exclusion Criteria: Patients whose disease relapsed during the first 2 years of adjuvant endocrine therapy (defined as primary ET resistance) were excluded.
Bibliographic Data
-
Title: Metronomic Capecitabine Plus Aromatase Inhibitor as Initial Therapy in Patients With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: The Phase III MECCA Trial
-
Authors: Ruo-Xi Hong, Fei Xu, Wen Xia, et al.
-
Journal: Journal of Clinical Oncology
-
Year: 2025 (Published online Jan 2, 2025)
-
DOI: 10.1200/JCO.24.00938
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text: PubMed Central
Attribution-NonCommercial-NoDerivatives 4.0 International
CC BY-NC-ND 4.0 Deed Canonical URL https://creativecommons.org/licenses/by-nc-nd/4.0/
