Comment:
This study provides crucial clarity on the fenbendazole story. The key takeaway is the stark difference between in vitro (lab dish) and in vivo (live animal) results. While FZ can kill cancer cells in a dish, this study demonstrates it completely fails in live models when given in its natural form.
The reason is simple: poor bioavailability. The drug isn’t water-soluble, so it cannot be absorbed into the bloodstream to reach the tumor, whether taken orally or even injected.
It’s critical to understand that the anti-cancer effects seen in this study were only achieved after the researchers developed a new, highly specialized nanoparticle formulation to overcome this absorption problem. The natural fenbendazole powder or suspension available over-the-counter does not have this property and was shown here to be ineffective.
Summary:
Clinical Bottom Line
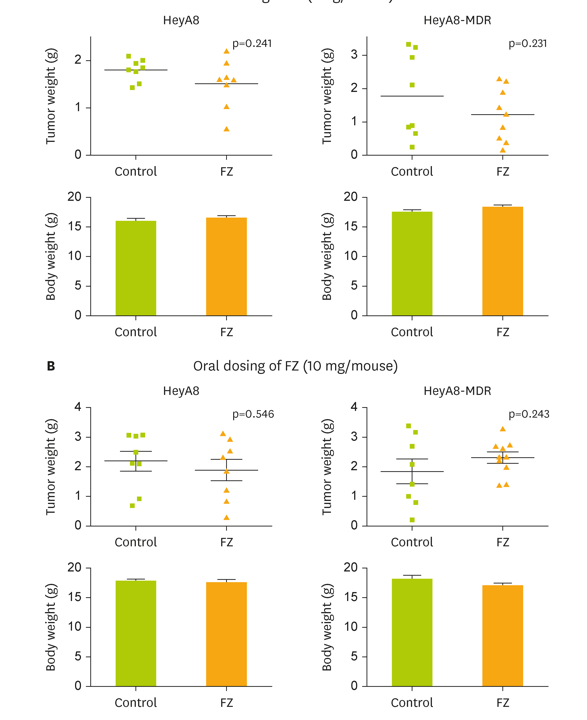
The study demonstrates that while natural fenbendazole (FZ) shows anti-cancer activity against epithelial ovarian cancer (EOC) cells in a lab dish (in vitro), it completely lacks efficacy in live mouse models (in vivo).
This failure is directly attributed to its poor water solubility, which prevents systemic absorption and makes it impossible to achieve therapeutic drug levels in the blood. Both oral and intraperitoneal administration of natural FZ failed to reduce tumors, leading the authors to conclude that the natural form cannot be effectively delivered to tumor cells in the body.
Thematic Analysis: Efficacy and Bioavailability
The study’s primary objective was to investigate FZ as a repurposed anti-cancer drug for EOC. A major hurdle identified from the outset was FZ’s poor water solubility, which limits its therapeutic use.
Failure of In Vivo Efficacy
While FZ effectively decreased cell proliferation in EOC cell lines in lab dishes, this effect did not translate to living models:
-
Oral Administration: In xenograft mouse models, oral FZ (at 1 mg/mouse and 10 mg/mouse doses) had no effect on tumor reduction compared to the control group. The paper notes this is likely due to low systemic bioavailability and extensive first-pass metabolism.
-
Intraperitoneal (IP) Administration: To bypass first-pass metabolism, the researchers tried injecting FZ directly into the peritoneal cavity. This also failed. The drug was not absorbed; instead, it aggregated in the intraperitoneal space due to its poor solubility.
The authors explicitly conclude that FZ in its natural form could not be delivered to the tumor cells in vivo.
Context and Implications
The study’s findings on the in vivo failure of natural FZ are presented as “scientific evidence against self-administration of FZ by cancer patients.”1
The anti-cancer effects observed in the study were only achieved after the researchers successfully developed a water-soluble nanoparticle formulation (FZ-PLGA-NPs) to overcome t2his critical bioavailability challenge. The natural, unaltered form of the drug was shown to be ineffective in vivo for treating EOC tumors.
This preclinical study provides strong evidence that natural fenbendazole is ineffective for treating EOC due to its physical properties that prevent systemic absorption and drug delivery to the tumor.
Analyzed Paper:
-
Title: Anti-cancer effect of fenbendazole-incorporated PLGA nanoparticles in ovarian cancer
-
Authors: Chang CS, Ryu JY, Choi JK, et al.
-
Journal: Journal of Gynecologic Oncology (2023)
- DOI: https://doi.org/10.3802/jgo.2023.34.e58
Original Article:
Full text pdf: Anti-cancer effect of fenbendazole-incorporated PLGA nanoparticles in ovarian cancer
Open Access
