Comment:
This is an important pre-clinical study, adding another piece to the connection between levothyroxine and cancer.
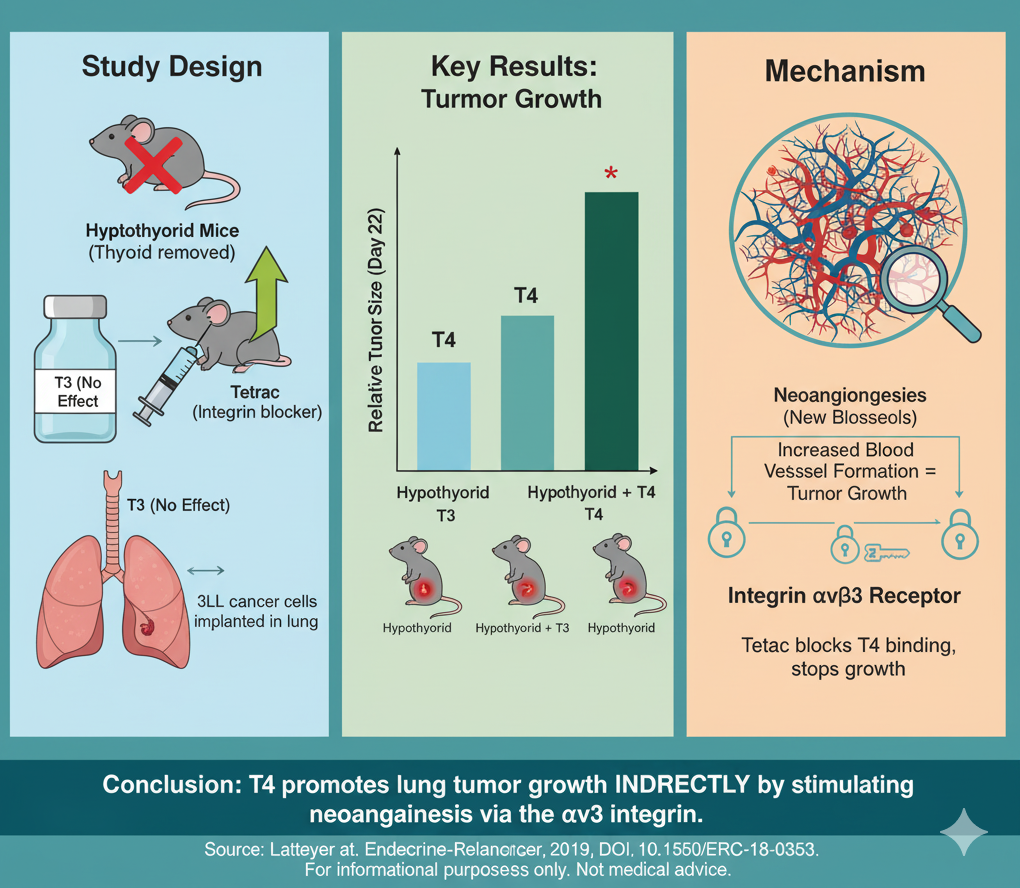
The finding that T4 promotes tumor growth not by direct cell stimulation, but by driving neoangiogenesis via the integrin αvβ3 receptor, is a critical piece of biologic plausibility.
Of course, the major caveat is that this is a mouse model. However, as another paper in a growing series (here, here) it further underscores the urgency of understanding what levothyroxine replacement really does in our cancer, especially those made hypothyroid by their TKI or checkpoint inhibitor therapy.
Summary:
Clinical Bottom Line:
This pre-clinical study in mice found that thyroxine (T4) promoted the growth of implanted lung cancers, while T3 did not. The mechanism was not direct stimulation of the cancer cells. Instead, T4 appeared to fuel tumor growth by promoting the formation of new blood vessels (neoangiogenesis), an effect mediated by the integrin αvβ3 cell surface receptor. This finding raises a hypothesis that levothyroxine substitution in hypothyroid cancer patients could potentially impact tumor progression, though this mouse data cannot be directly extrapolated to humans.
Results in Context
Research Objective: The study’s goal was to see if thyroid hormones (T4 and T3) promote lung cancer growth in a live mouse model (orthotopic) and to investigate the mechanism, specifically the role of the integrin αvβ3 receptor.
Study Design and Methods:
-
In Vivo (Mouse) Model: Researchers injected murine Lewis lung carcinoma (3LL) cells directly into the lungs of mice. They then created several mouse groups: euthyroid (normal thyroid function), hypothyroid, hypothyroid + T3 replacement, and hypothyroid + T4 replacement.
-
Inhibitor Arm: To test the mechanism, some groups also received Tetrac, a compound that blocks T4 and T3 from binding to the integrin αvβ3 receptor.
-
Measurements: Tumor growth was tracked over time using bioluminescence imaging. After the experiment, tumors were weighed and analyzed for new blood vessel density (neoangiogenesis) using a CD31 stain.
-
In Vitro (Cell) Model: The 3LL cancer cells were also studied in a lab dish to see if T4 directly stimulated their proliferation or activated key cancer-promoting pathways (Akt and Erk).
Key Findings:
-
Tumor Growth: Compared to normal (euthyroid) mice, tumor growth was reduced in hypothyroid mice.
-
T4 vs. T3 Effect: Treating hypothyroid mice with T4 significantly increased tumor growth and final tumor weight. In contrast, treating with T3 had no effect on tumor growth.
-
Mechanism of Action:
-
The T4-driven tumor growth was completely abrogated (blocked) by co-treatment with the integrin αvβ3 inhibitor, Tetrac.
-
In the lab dish, T4 did not directly stimulate the cancer cells to proliferate or activate the Akt/Erk signaling pathways.
-
Analysis of the tumors showed that T4 treatment led to a significant increase in neoangiogenesis (new blood vessels), and this effect was also blocked by Tetrac.
-
Assertive Critical Appraisal
Conclusions: The authors conclude that in this specific mouse model, T4 promotes lung cancer growth. This effect is not from T4 directly stimulating the cancer cells but is an indirect effect mediated through the integrin αvβ3 receptor, which leads to increased blood vessel formation, supplying the tumor with more nutrients.
Clinical Relevance & Limitations:
-
Translational Hypothesis: This is a well-conducted pre-clinical study that provides a plausible biologic mechanism. Its primary value is in generating a hypothesis for human studies. The authors note that many cancer patients become hypothyroid as a side effect of treatments (like checkpoint inhibitors or tyrosine kinase inhibitors) and require levothyroxine (T4) substitution. This study suggests, but does not prove, that T4 substitution in this context might need to be considered more carefully.
-
Critical Weakness: This is a mouse model. The findings cannot be directly applied to clinical practice. Human lung cancer biology and the human endocrine system are far more complex.1
-
Endpoint: The study measured tumor growth and neoangiogenesis, not survival. While tumor growth was affected, overall survival in the mice was not different between the 2groups.
Bibliographic Data
-
Title: Thyroxine promotes lung cancer growth in an orthotopic mouse model
-
Authors: S Latteyer, S Christoph, S Theurer, GS Hönes, K W Schmid, D Führer and L C Moeller
-
Journal: Endocrine-Related Cancer
-
Year: 2019
-
DOI:
https://doi.org/10.1530/ERC-18-0353
This analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text : here

