Comment:
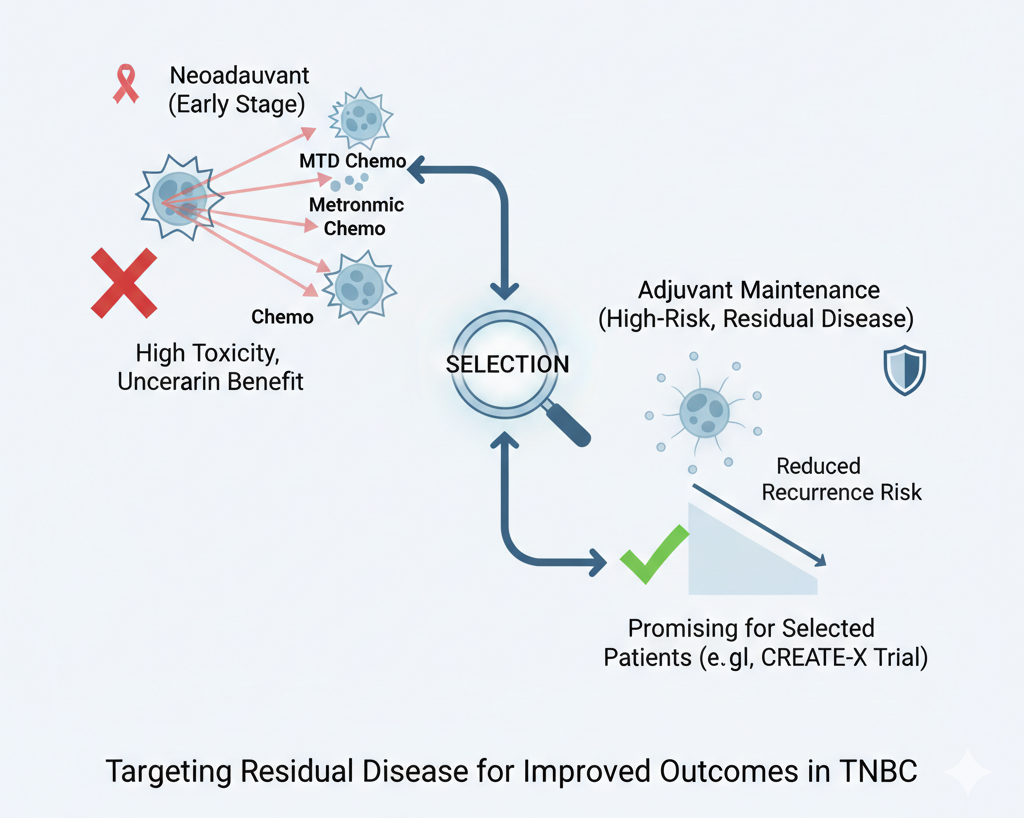
This paper tries to identify the true clinical niche for Metronomic Chemotherapy: adjuvant maintenance therapy for patients with residual disease. This is the high-risk subgroup where the gold standard, maximum tolerated dose (MTD) chemotherapy, has clearly failed to eradicate the disease. The success of the CREATE-X trial in this specific, defined population—with its remarkable 42% risk reduction in the TNBC subset—is the definitive signal. It validates the concept of leveraging MC’s anti-angiogenic and immune-modulating properties as a sustained “mop-up” strategy where MTD cannot safely continue.
Summary:
🏥 Clinical Bottom Line
This review explores metronomic chemotherapy (MC)—the frequent administration of low-dose chemo—as a strategy for non-metastatic triple-negative breast cancer (TNBC). The authors conclude that while neoadjuvant MC regimens can achieve high rates of pathologic complete response (pCR), they are associated with significant toxicity. The most promising role for MC appears to be as an adjuvant maintenance therapy for high-risk patients, particularly those who have residual disease (i.e., did not achieve a pCR) after standard neoadjuvant treatment. The authors emphasize that future clinical trials must focus on this specific, high-risk population to validate the benefit of MC.
📝 Summary of Key Findings
This article reviews the rationale for MC and its application in TNBC, contrasting it with standard maximum tolerated dose (MTD) chemotherapy.
-
Rationale for MC: Unlike MTD chemotherapy, which targets rapidly proliferating tumor cells, MC is thought to work through multiple mechanisms. These include inhibiting angiogenesis (targeting tumor blood vessels) and activating an immune response (e.g., by reducing inhibitory T-reg cells).
-
Neoadjuvant Setting: Standard MTD chemotherapy for TNBC yields pCR rates between 20% and 39%. The review notes that several trials of neoadjuvant MC (using “metronomic-only” or “hybrid” MTD-MC approaches) achieved much higher pCR rates, ranging from 47% to 60%. However, this efficacy came at the cost of great toxicity, including high rates of Grade 3-4 neutropenia.
-
Adjuvant Setting (Intensification): The review states that attempts to improve outcomes by simply intensifying standard adjuvant MTD regimens with MC have failed to show a benefit.
-
Adjuvant Setting (Maintenance): The authors identify maintenance therapy as a “more promising” use of MC. This approach is specifically for high-risk patients, such as those with residual disease after neoadjuvant therapy. The review highlights the CREATE-X trial, which randomized HER2-negative patients with residual disease to capecitabine maintenance or observation. This trial demonstrated a significant 30% reduction in the risk of recurrence, with the benefit being even greater (a 42% risk reduction) in the TNBC subgroup.
🔬 Authors’ Critical Appraisal and Future Outlook
The authors provide a critical perspective on the available data, emphasizing that “selection is the key”.
-
On Neoadjuvant Data: The authors caution that the high pCR rates in the neoadjuvant MC trials are difficult to interpret. They point out that all but one of these studies also incorporated platinum salts. Therefore, the positive results cannot be attributed to the metronomic schedule alone and should be compared against other platinum-containing MTD regimens. The significant toxicity also remains a major limitation.
-
On Adjuvant Data: The review contrasts the positive CREATE-X trial with the negative IBCSG Trial 22. The authors praise the CREATE-X study for correctly identifying residual disease as a biomarker of high risk and selecting this population for maintenance therapy. They criticize the IBCSG trial for including a broad population (not selected for risk) and for using varied and older chemotherapy backbones, which modified the outcomes.
-
Future Direction: The authors strongly conclude that future research should abandon a “one-size-fits-all” approach. They advocate for trials that specifically enroll high-risk TNBC patients, such as those with residual disease after neoadjuvant treatment, to confirm the benefits of MC maintenance. They note that ongoing trials, like the ECOG-ACRIN EA 1131, are properly designed to answer this question by testing capecitabine or platinum against observation in this exact patient population.
🎯 Research Objective
The objective of this minireview was to outline the rationale, preclinical data, and relevant clinical trials for metronomic chemotherapy (MC) as an alternative strategy to improve outcomes for patients with non-metastatic triple-negative breast cancer (TNBC).
📖 Study Design
This article is a minireview. It summarizes the mechanisms of MC and appraises the results of clinical trials that have tested MC in the neoadjuvant and adjuvant settings (both as intensification and maintenance therapy) for TNBC.
👥 Population of Focus
The review focuses on non-metastatic triple-negative breast cancer (TNBC), defined as a molecularly heterogeneous and aggressive disease that accounts for 15%-20% of all breast cancers. The authors note that TNBC is still the only breast cancer subtype for which there are no approved targeted therapies.
📚 Bibliographic Data
-
Title: Metronomic chemotherapy for non-metastatic triple negative breast cancer: Selection is the key
-
Authors: Connie Rabanal, Rossana Ruiz, Silvia Neciosup, Henry Gomez
-
Journal: World Journal of Clinical Oncology
-
Year: 2017
-
DOI: 10.5306/wjco.v8.i6.437
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text pdf: Metronomic chemotherapy for non-metastatic triple negative breast cancer Selection is the key
This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Rabanal C, Ruiz R, Neciosup S, Gomez H. Metronomic chemotherapy for non-metastatic triple negative breast cancer: Selection is the key. World J Clin Oncol 2017; 8(6): 437-446 [PMID: 29291168 DOI: 10.5306/wjco.v8.i6.437]
