Comment:
This meta-analysis provides important, ‘Moderate’ certainty evidence that supports a clinical approach that’s simple to implement. The key takeaway is the non-inferiority of intermittent androgen deprivation (IAD) regarding overall survival.
While the summary notes minimal difference in overall quality of life, the qualitative data suggesting improvements in physical and sexual functioning is clinically significant. This directly addresses the primary goal of IAD: to provide patients with a “drug holiday” to mitigate the debilitating cumulative side effects of continuous therapy.
The oncologic outcomes (OS, CSS, PFS) are statistically equivalent. Therefore, the decision between IAD and CAD should pivot on the adverse effect profile and patient preference. Given the well-known toxicities of continuous androgen deprivation—including metabolic syndrome, bone density loss, and severe fatigue—the IAD approach presents a much lower-risk and more patient-centric alternative.
From the clinical side, it provides us the opportunity to use naturopathic therapies to extend the time between doses, making it even more intermittent 😊.
Summary:
🩺 Clinical Bottom Line
This meta-analysis suggests that intermittent androgen deprivation therapy (IAD) is non-inferior to continuous androgen deprivation (CAD) regarding overall survival for patients with prostate cancer. The certainty of this evidence was rated as ‘Moderate’. While the analysis found minimal difference in overall quality of life between the two approaches, some evidence suggests IAD may improve specific domains, particularly physical and sexual functioning. This makes IAD a reasonable therapeutic alternative, but this conclusion must be balanced against the fact that nearly all trials included in the analysis had an unclear or high risk of bias.
📊 Results
-
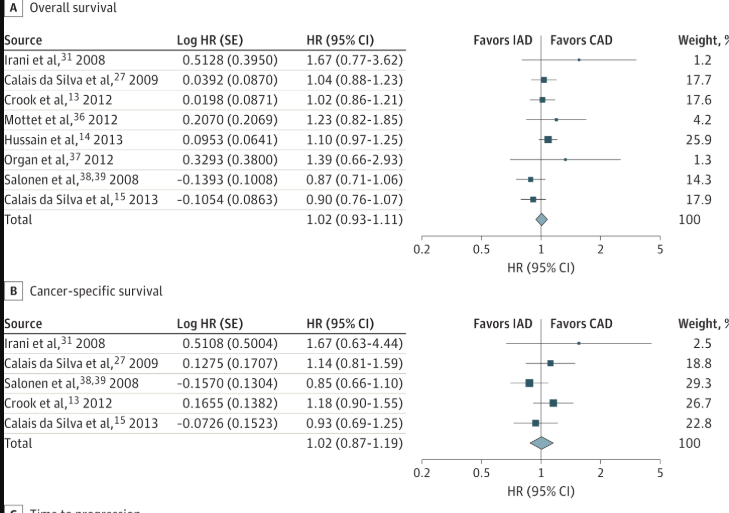
Overall Survival (OS): The pooled analysis of 8 trials (5,352 patients) found no significant difference between intermittent and continuous therapy (Hazard Ratio [HR], 1.02; 95% CI, 0.93-1.11).
-
Definition: A hazard ratio (HR) compares the rate of an event (like death) in two groups. An HR of 1.02 means the rate of death in the IAD group was 2% higher than in the CAD group, a difference considered statistically non-significant as the 95% CI includes 1.0.
-
Non-Inferiority Finding: The authors pre-specified a non-inferiority margin of 1.15. Because the upper end of the confidence interval (1.11) was below this margin, the study’s hypothesis that IAD is non-inferior to CAD for overall survival was supported.
-
Cancer-Specific Survival (CSS): No significant difference was found based on 5 trials (3,613 patients) (HR, 1.02; 95% CI, 0.87-1.19).
-
Progression-Free Survival (PFS): No significant difference was found based on 4 trials (1,774 patients) (HR, 0.94; 95% CI, 0.84-1.05).
-
Quality of Life (QoL): A formal meta-analysis was not possible due to the different measurement tools used and unavailable data. Qualitatively, the review found “minimal difference” in patients’ self-reported QoL. However, most trials observed an improvement in physical and sexual functioning with intermittent therapy.
-
Adverse Effects: While pooled point estimates tended to favor IAD, no significant difference was found for reported drug-related adverse effects.
🧐 Assertive Critical Appraisal
-
Certainty of Evidence (GRADE Framework): The authors assessed the overall strength of evidence for the primary outcome, overall survival, as ‘Moderate’. The main reason for not rating it ‘High’ was the poor methodological quality of the included trials. The evidence for quality of life was considered weak, given a high summary risk of bias.
-
Risk of Bias in Included Studies: This is the most significant weakness of the evidence base. Of the 15 unique trials included, 14 were judged to have an “unclear or high risk of bias”. Key issues included 6 trials not being blinded (with 9 others being unclear), high withdrawal rates in some studies, and post-randomization exclusion of patients from the analysis.
-
Heterogeneity: The authors used the I² statistic to assess for differences between studies.
-
Definition: The I² statistic describes the percentage of variation across studies that is due to genuine differences in effect rather than just chance.
-
Heterogeneity was low for overall survival (I² = 23%) and cancer-specific survival (I² = 14%), and absent for progression-free survival (I² = 0%). This consistency strengthens the reliability of these specific pooled results.
-
-
Publication Bias: The authors visually inspected a funnel plot for the primary outcome and did not find evidence of publication bias.
-
Reporting Quality Assessment (PRISMA): This systematic review itself is well-reported and follows the PRISMA guidelines. It includes a PRISMA flow diagram (Figure 1) detailing the study selection process and describes its comprehensive search strategy.
🎯 Research Objective
-
PICO Question:
-
Population: Patients with prostate cancer (any stage).
-
Intervention: Intermittent androgen deprivation therapy (IAD).
-
Comparison: Continuous androgen deprivation therapy (CAD).
-
Outcomes: Primary outcomes were overall survival and quality of life.
-
Hypothesis: The authors hypothesized that IAD is non-inferior to CAD for overall survival.
-
DESIGN
-
Study Design: A systematic review and meta-analysis of randomized clinical trials.
-
Search Strategy: The authors searched multiple databases (including Cochrane CENTRAL, Medline, and Embase) and gray literature sources from their inception through March 2014.
-
Included Studies & Participants: After screening 10,510 references, the authors included 22 articles that reported on 15 unique randomized trials.
-
Total Participants: The analysis included a total of 6,856 patients. The mean age of participants was 70 years.
📄 Bibliographic Data
-
Title: Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis
-
Authors: Sindy Magnan, Ryan Zarychanski, Laurie Pilote, Laurence Bernier, Michèle Shemilt, Eric Vigneault, Vincent Fradet, Alexis F. Turgeon
-
Journal: JAMA Oncology
-
Year: 2015
-
DOI: 10.1001/jamaoncol.2015.2895
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical cond1ition.
Original Article:
Full text pdf: Available here.
.
