Comment:
This 2017 article’s real contribution here isn’t the narrative review—which, as is too selective to be high-quality—but the new VICTOR-2 subgroup data. While a 35.7% Clinical Benefit Rate in an elderly (median age 69) metastatic TNBC population is certainly interesting, it’s from a tiny, single-arm cohort of 28 patients.
This paper highlights metronomic chemotherapy as a potential palliative option, especially for frail or elderly patients where standard, max-dose chemo is not a viable option due to toxicity.
Summary:
Clinical Bottom Line
This 2017 article by Cazzaniga et al. provides a narrative review of the sparse preclinical and clinical data available for metronomic chemotherapy (mCHT) in triple-negative breast cancer (TNBC). The authors conclude that while evidence is very limited—drawn mostly from small Phase II trials or subgroup analyses—some promising results exist, particularly as a potential treatment option for specific populations like the elderly.
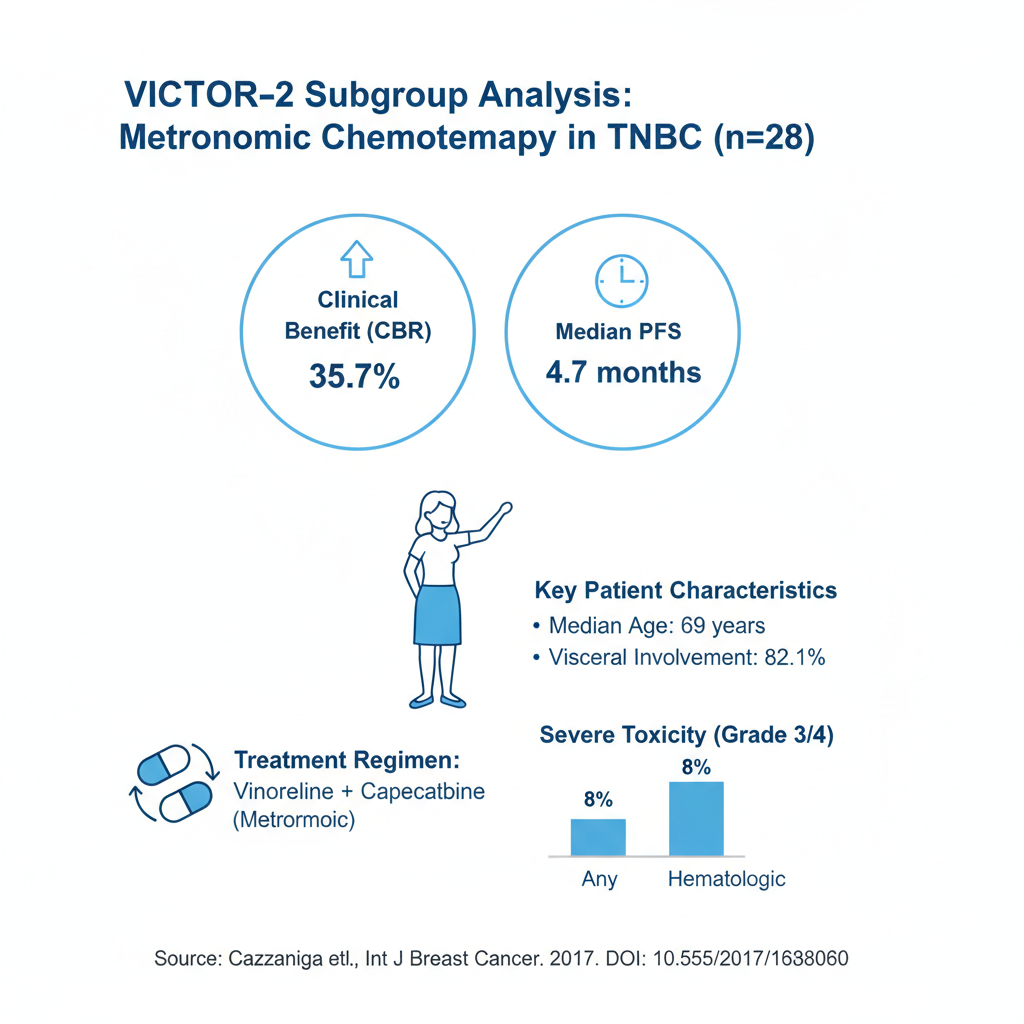
Crucially, this paper is not a systematic review and its conclusions are based on a selective literature search. Its most novel contribution is the first-time reporting of prospective data from the TNBC subgroup (n=28) of the authors’ own VICTOR-2 Phase II trial, which showed a clinical benefit rate of 35.7% with a metronomic combination of vinorelbine and capecitabine.
Results
Metronomic chemotherapy is defined as the minimum biologically effective dose of a chemotherapy agent given continuously with no prolonged drug-free breaks. The review summarizes data across three setting1s:
Preclinical Data
-
One study in a TNBC mouse model found that metronomic topotecan combined with pazopanib significantly enhanced antitumor activity and prolonged survival compared to either drug alone.
(Neo)Adjuvant Setting
-
The review highlights mixed results from a few trials.
-
The IBCSG 22-00 trial found no statistically significant benefit for metronomic cyclophosphamide and methotrexate (CM) maintenance in the overall TNBC population.
-
In contrast, a study by Nasr et al. concluded that extended adjuvant metronomic CM did achieve a significant improvement in survival and was well-tolerated.
-
The authors of this review state their opinion that mCHT should not be adopted in the adjuvant setting without stronger preclinical data.
Metastatic Setting
-
Data is noted as being very scarce, with most trials enrolling HER2-negative patients and reporting on TNBC patients only in subgroup analyses.
VICTOR-2 Subgroup Analysis (New Data)
The review presents new data on 28 TNBC patients from the VICTOR-2 study, a Phase II, single-arm trial evaluating metronomic vinorelbine (40 mg three times/week) and capecitabine (500 mg three times/day).
-
Population: Median age was 69 years. 82.1% had visceral involvement.
-
Efficacy:
-
Clinical Benefit Rate (CBR): 35.7%
-
Disease Control Rate (DCR): 53.6%
-
Median PFS: 4.7 months
-
-
Toxicity: Severe (Grade 3/4) toxicity did not exceed 8% and was mainly hematologic.
Critical Appraisal
This article is a hybrid narrative review and primary data report. Its structure has significant limitations as a review.
-
Certainty of Evidence: The overall certainty of the evidence presented for mCHT in TNBC is Very Low. This rating is based on the review’s reliance on a small number of preclinical studies, subgroup analyses, and small, non-randomized Phase II studies.
-
Search Strategy & Reporting: The article does not follow PRISMA guidelines for systematic reviews.
-
Search Strategy: This is a significant limitation. The search was restricted to only one database (PubMed) using three keywords, creating a high risk of selection bias.
-
Bias Assessment: The authors do not provide a formal risk of bias assessment for the included studies.
-
-
Primary Contribution: The review itself is limited. The article’s most significant contribution is the inclusion of the first prospective data (though from a small, single-arm subgroup) on metronomic vinorelbine and capecitabine in metastatic TNBC patients.
Article Citation
-
Title: Metronomic Chemotherapy in Triple-Negative Metastatic Breast Cancer: The Future Is Now?
-
Authors: M. E. Cazzaniga, L. Cortesi, A. Ferzi, L. Scaltriti, F. Cicchiello, M. Ciccarese, S. Della Torre, F. Villa, M. Giordano, C. Verusio, M. Nicolini, A. R. Gambaro, L. Zanlorenzi, E. Biraghi, E. Casini, L. Legramandi, and E. Rulli
-
Journal: International Journal of Breast Cancer
-
Year: 2017
Disclaimer: This analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text pdf: Metronomic Chemotherapy in Triple-Negative Metastatic Breast Cancer The Future Is Now
Open Access
To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
