Comment:
Unfortunately, this study confirms a persistent issue. We repeatedly see this major discrepancy between the “known unknowns”—the clinical uncertainties the FDA rightly identifies during expedited approvals—and what actually gets published in high-impact journals and translated into clinical guidelines like the NCCN.
The result is that frontline oncologists are often making treatment decisions with an incomplete, and overly optimistic, data set. They may be unaware of significant limitations, such as reliance on single-arm trials or unvalidated surrogate endpoints, simply because those limitations weren’t reported in the sources they trust.
It’s a systemic failure that directly impacts patient care. Until journal editors and guideline committees take responsibility for reporting these FDA-flagged uncertainties transparently, clinicians will continue to overestimate benefits and underestimate risks.
Summary:
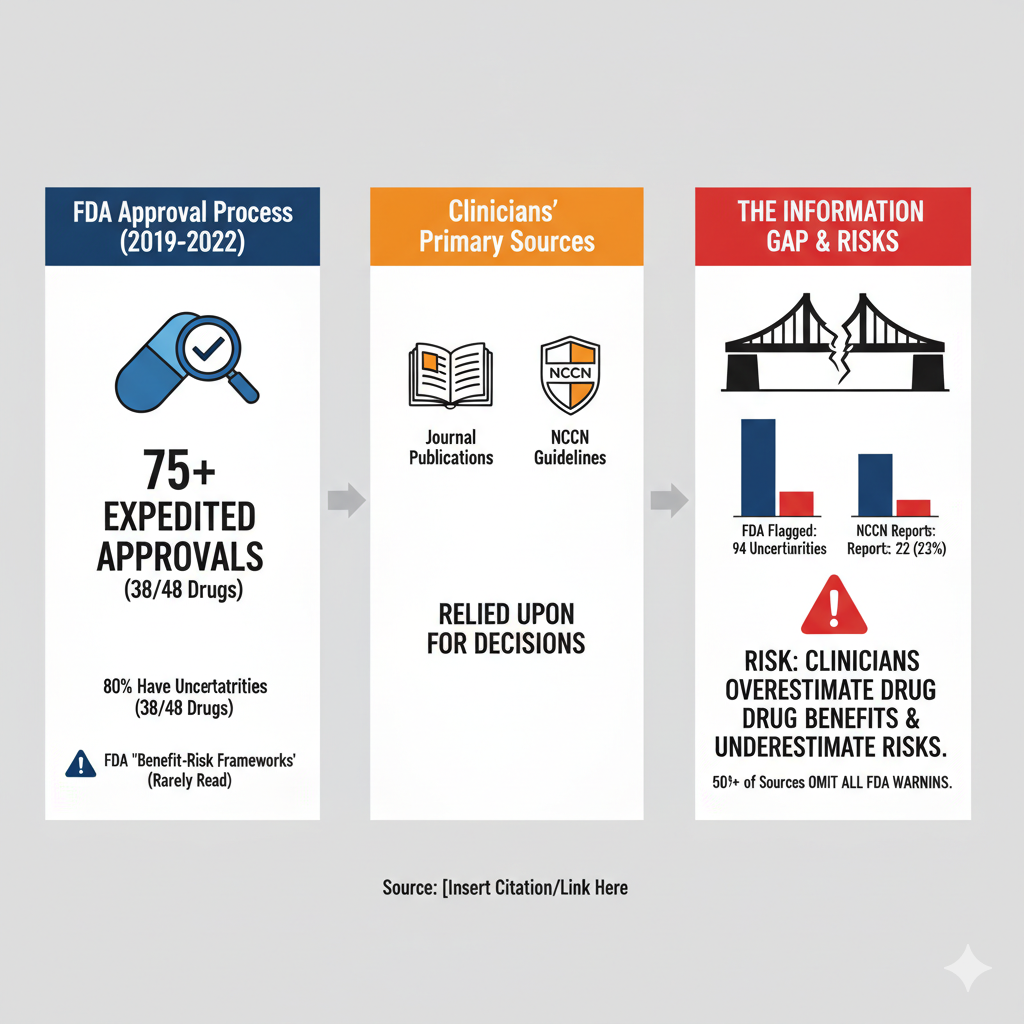
Thesis: A Critical Gap in Reporting Risks
The central argument is that a dangerous discrepancy exists between the known “clinical uncertainties” of new cancer drugs, as identified by the FDA, and the information that is actually reported in the journal publications and clinical guidelines that oncologists use for decision-making.
This reporting failure creates a significant risk: clinicians, unaware of the trial’s limitations, are likely to “overestimate a drug’s benefits and underestimate its risks”.
Core Tension and Key Findings
The study identifies a fundamental conflict:
-
The Problem: More than 75% of cancer drugs are approved through expedited FDA programs, which often leaves “clinical uncertainties” about their true efficacy and safety. These can include issues like unvalidated endpoints, limited long-term data, or reliance on single-arm trials.
-
The Information Gap: The FDA details these uncertainties in its “Benefit-Risk Frameworks,” but clinicians rarely read these documents. Instead, they rely on pivotal trial publications and NCCN guidelines to guide treatment.
-
The Finding: The authors investigated whether these key sources actually report the uncertainties the FDA identified. They found they do not.
The analysis of cancer drugs approved from 2019-2022 revealed:
-
Widespread Uncertainties: Nearly 80% (38 of 48) of the approved drugs had clinical trial uncertainties highlighted by the FDA.
-
Poor Reporting: Journal publications reported only 22% (21/94) of these FDA-identified uncertainties. NCCN guidelines were almost identical, reporting only 23% (22/94).
-
Complete Omission: More than half of the journal publications (53%) and 47% of the NCCN guidelines did not report any of the uncertainties the FDA had flagged for those drugs.
🩺 Risks and Overestimation of Efficacy
The “so what” of this research is that the primary information sources for oncologists are presenting an incomplete, and likely overly positive, picture of new drugs.
-
Clinicians Are Unaware: The authors state this discrepancy “suggests that clinicians may be unaware of important clinical trial limitations… when making prescribing decisions”.
-
Common, Unreported Risks: The most common uncertainties identified by the FDA included “Long-term benefits and harms” (23 instances) and “Single-arm trial design” (22 instances). These specific, critical limitations are what clinicians are not being told about, as they were reported less than 30% of the time in either journals or guidelines.
-
False Confidence: By omitting these FDA-identified concerns, publications and guidelines inadvertently encourage an overestimation of the drug’s benefit-risk balance. A clinician, reading the guideline or publication, would not have the necessary context to critically appraise the drug’s true value or discuss its limitations fully with a patient.
Original Article:
Full text pdf: Available at JAMA site pdf link here.
