Comment:
This large pooled analysis gives us several interesting and important pieces of information. Although this study was with breast cancer patients, there is no reason to believe it doesn’t apply to every cancer.
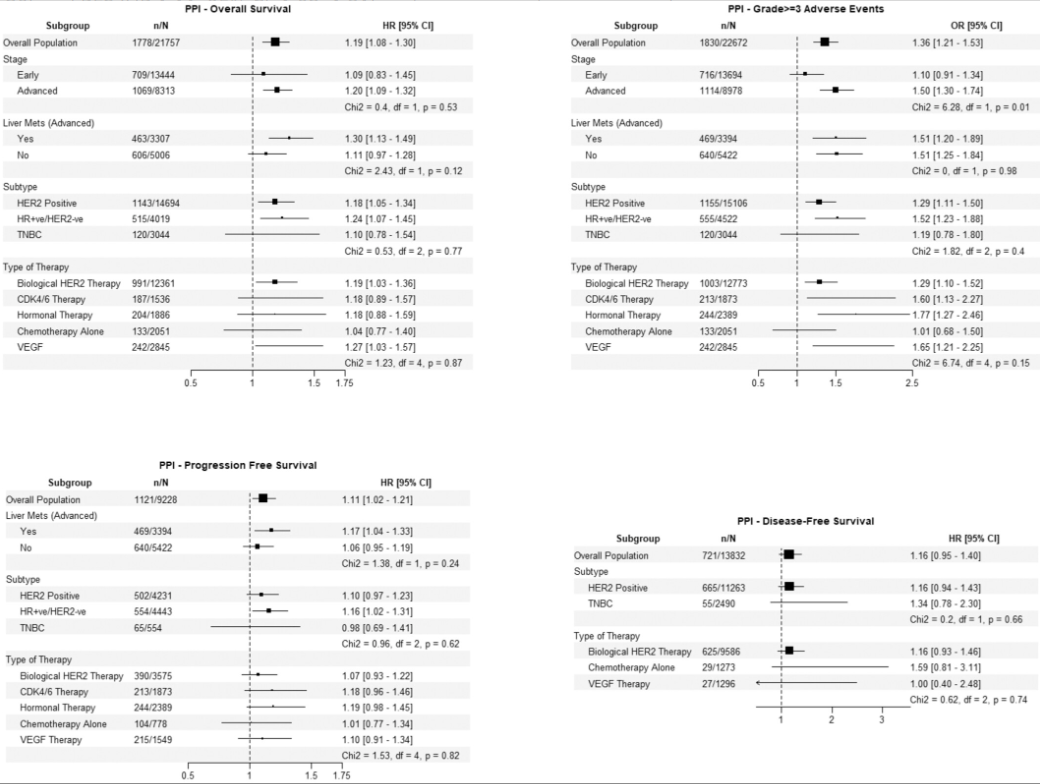
The magnitude of risk associated with common, non-oncology drugs is genuinely alarming. PPI use, a near-universal practice for GI prophylaxis and symptom management, was linked to a 19% increase in the hazard of death and a 36% increased odds of severe adverse events. Similarly, key cardiovascular medications like beta-blockers, ACE inhibitors/ARBs and CCBs were clearly associated with higher rates of Grade ≥3 toxicity.
Second, the paper looked at statins and metformin—two of the most heavily hyped and frequently studied repurposed cancer agents. They showed no significant association with either survival or adverse events. The null result here, derived from over 23,000 individual patient records, strongly suggests that the widely celebrated epidemiological signals for these drugs may be heavily driven by confounding or limited by prior smaller-scale studies.
Summary:
Clinical Bottom Line
This large, pooled analysis of individual patient data from 19 breast cancer clinical trials investigated the impact of common non-cancer medications on patient outcomes . The most significant finding was that proton pump inhibitor (PPI) use was associated with poorer overall survival, poorer progression-free survival, and an increased risk of severe (grade ≥3 ) adverse events .
Common cardiovascular drugs—specifically beta-blockers, ACE inhibitors/ARBs, and calcium channel blockers—were also associated with a higher risk of grade ≥3 adverse events , though they showed no significant impact on survival . Reassuringly, statins and metformin were not associated with any significant negative (or positive) impact on survival or adverse events .
As this is an observational study, these findings show strong associations but cannot prove causation . The associations, especially for PPIs, warrant careful consideration and monitoring in clinical practice .
Results in Context: Risks by Medication Class
This study analyzed data from 23,211 breast cancer patients and adjusted for factors including age, BMI, cancer subtype, performance status, and comorbidity count . The risks identified for each medication class were as follows:
Proton Pump Inhibitors (PPIs)
-
Poorer Overall Survival (OS): Associated with a 19% increase in the hazard of death (HR 1.19, 95% CI: 1.08-1.30) .
-
Poorer Progression-Free Survival (PFS): Associated with an 11% increase in the hazard of progression or death (HR 1.11, 95% CI: 1.02-1.21) .
-
Increased Adverse Events: Associated with a 36% increased odds of experiencing grade ≥3 adverse events (OR 1.36, 95% CI: 1.21-1.53) .
Beta-Blockers (BBs)
-
Increased Adverse Events: Associated with a 21% increased odds of grade ≥3 adverse events (OR 1.21, 95% CI: 1.07-1.36) .
-
No Survival Impact: Showed no significant association with overall survival, progression-free survival, or disease-free survival .
ACE Inhibitors / ARBs
-
Increased Adverse Events: Associated with a 13% increased odds of grade ≥3 adverse events (OR 1.13, 95% CI: 1.01-1.26) .
-
No Survival Impact: Showed no significant association with survival outcomes .
Calcium Channel Blockers (CCBs)
-
Increased Adverse Events: Associated with a 31% increased odds of grade ≥3 adverse events (OR 1.31, 95% CI: 1.13-1.51) .
-
No Survival Impact: Showed no significant association with survival outcomes .
Statins and Metformin
-
No Significant Associations: Use of statins or metformin was not significantly associated with any difference in survival (OS, PFS, DFS) or the risk of grade ≥3 adverse events .
Assertive Critical Appraisal
-
Limitations & Bias (STROBE Framework): The primary limitation, clearly stated by the authors, is that this is a post-hoc observational study . Therefore, it cannot establish a definitive causal relationship between these drugs and the observed outcomes .
-
Risk of Confounding: The associations found could be due to residual confounding . For instance, patients prescribed PPIs may have other underlying health issues, symptoms (like GORD or dyspepsia ), or be taking other medications (like steroids ) that are the true cause of their poorer outcomes, rather than the PPI itself. While the analysis adjusted for the number of comorbidities and the common reasons for PPI use, this risk can never be fully eliminated in an observational design .
-
Missing Data: The analysis lacked detailed data on medication dosing, duration of use, polypharmacy, and patient adherence, all of which could influence the results .
Research Objective
The study’s aim was to evaluate the associations between the use of common non-cancer concomitant medications and survival outcomes (OS, PFS, DFS) as well as the risk of grade ≥ adverse events in patients with breast cancer .
Study Design
This was a large-scale pooled individual participant data (IPD) meta-analysis , which combined data from 19 different breast cancer clinical trials . This observational design used statistical models (Cox proportional hazards and logistic regression) to find associations .
Setting and Participants
The study included a total of 23,211 participants from 19 breast cancer clinical trials . The population was diverse, including 13,837 (60%) patients with early-stage breast cancer and 9,374 (40%) with advanced breast cancer .
Bibliographic Data
-
Title: Associations of Commonly Used Concomitant Medications With Survival and Adverse Event Outcomes in Breast Cancer
-
Authors: Natansh D. Modi, Ahmad Y. Abuhelwa, Nicole M. Kuderer, Lee X. Li, Gary H. Lyman, Bogda Koczwara, Ganessan Kichenadasse, Luke A. Selth, Ceara Rickard, Mark Haseloff, Agnes Vitry, Richard Woodman, Jessica M. Logan, Huah Shin Ng, Michael D. Wiese, Ross A. McKinnon, Andrew Rowland, Michael J. Sorich, Ashley M. Hopkins
-
Journal: Cancer Medicine
-
Year: 2025
-
DOI: [https://doi.org/10.1002/cam4.71320]
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text pdf: Cancer Medicine – 2025 – Modi – Associations of Commonly Used Concomitant Medications With Survival and Adverse Event
Attribution 4.0 International CC BY 4.0
≥
