Comment:
This small but powerful study on dietary flaxseed is a great reminder that simple, well-tolerated dietary interventions can have profound biological effects in breast cancer. It also helps address a persistent misinterpretation, which is that flax can act negatively in breast cancer patients.
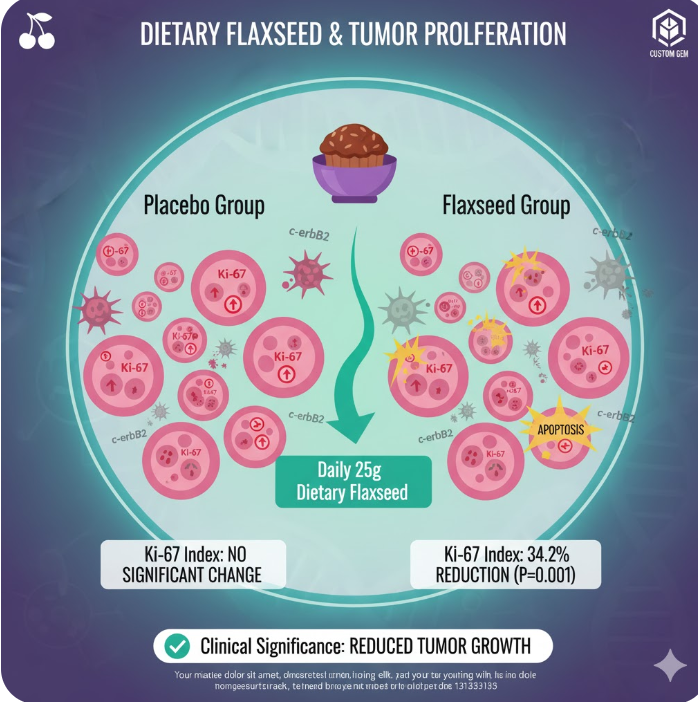
The study shows a significant reduction in the Ki-67 index (cell proliferation) by 34.2%, which is a crucial prognostic marker, alongside a massive 71% drop in the aggressive c-erbB2 marker and a concurrent 30.7% boost in apoptosis (cell death).
The fact that this benefit comes from just 25g of daily ground flaxseed, which is cheap, accessible, and has no systemic adverse events (just a bit of fiber-induced fullness), makes it incredibly attractive. While we of coruse need larger, longer-term trials on clinical outcomes, this data provides compelling biologic plausibility to consider flaxseed as a highly valuable dietary adjunct for postmenopausal breast cancer patients. It’s a low-risk, high-potential addition to therapy.
Summary:
🍒 Clinical Bottom Line
This short-term, randomized, double-blind, placebo-controlled clinical trial in newly diagnosed postmenopausal breast cancer patients provides preliminary evidence that daily dietary flaxseed (25g) can favorably alter tumor biological markers associated with reduced tumor growth and a potentially less aggressive cancer phenotype. Specifically, flaxseed significantly reduced tumor cell proliferation (Ki-67 index) and the expression of the aggressive marker c-erbB2, while increasing apoptosis (programmed cell death) in the tumor tissue. The intervention was well-tolerated, with the only reported side effects being mild gastrointestinal discomfort due to the high fiber content. However, this was a small, short-duration study that assessed surrogate biomarkers rather than long-term clinical outcomes (like recurrence or survival), and these positive findings require confirmation in larger, longer-term trials before definitive clinical recommendations can be made.
Results in Context
Primary Outcome
The primary endpoint was the Ki-67 labeling index, a marker that measures the rate of tumor cell proliferation.
-
Result: The flaxseed group showed a statistically significant reduction of 34.2% (median) in the Ki-67 labeling index after treatment (P = 0.001). This reduction was not significant in the placebo group (P = 0.305).
-
Clinical Significance: Ki-67 reduction can be taken as a surrogate end point biomarker for the efficacy of endocrine agents in breast cancer treatment, based on the principle that efficacy is dependent on the successful induction of the arrest of cell proliferation. The 34.2% decrease observed with flaxseed was lower than the 46.4% reduction seen in a comparable study using tamoxifen for a median of 21 days.
Key Secondary & Specialized Outcomes
-
Apoptosis (Cell Death): The apoptotic index (percentage of positive cells over total cells) significantly increased by 30.7% (median) in the flaxseed group (P = 0.007), but not in the placebo group (P = 1.000).
-
c-erbB2 Expression (HER2): The expression of c-erbB2 (a marker associated with aggressive breast cancer phenotypes) significantly decreased by 71.0% (median) in the flaxseed group (P = 0.003), but not in the placebo group (P = 0.381).
-
Dose Correlation: The total intake of flaxseed was significantly correlated with both the changes in c-erbB2 score (r = -0.373; P = 0.036) and the apoptotic index (r = 0.495; P < 0.004).
-
Urinary Lignans: There was a significant 1,300% increase in mean urinary lignan excretion in the flaxseed group (P < 0.01), compared with placebo controls.
-
Estrogen/Progesterone Receptors: No significant differences were found between the pre- and post-treatment periods in any group for Estrogen Receptor (ER) or Progesterone Receptor (PR) levels.
Harms and Safety
-
Safety: Flaxseed was well-tolerated.
-
Adverse Events: The only side effects reported by subjects were abdominal fullness and increased bowel movements, attributed to the high fiber content of flaxseed. The authors note that an increase in bowel movement may be considered desirable for patients with low fiber intake and chronic constipation. No adverse effects on major organs were observed in supporting animal studies.
Assertive Critical Appraisal
Risk of Bias (RoB 2 Framework)
-
Overall Risk of Bias: Some concerns.
The trial was a randomized, double-blind, placebo-controlled study, which appropriately minimizes performance and ascertainment bias. The use of isocaloric muffins balanced for fat, protein, and dietary fiber helped to isolate the effect of flaxseed and maintain blinding. Furthermore, the pre- and post-treatment tumor tissue sections were stained and read by a reader who was blinded to the treatment group and whether the sample was pre- or post-treatment.
The main concern is the very small sample size (n = 32 completed patients) and the short duration of the intervention (mean of 32 days for the flaxseed group). While the study was designed to achieve 80% power for a 30% difference in Ki-67 change between groups, the small number of participants limits the precision of the estimates and the ability to generalize the results.
Biomarker Appraisal
The study appropriately selected prognostic biomarkers (Ki-67, c-erbB2, Apoptosis) as surrogate endpoints for a short-term preoperative trial, which is standard practice in the neoadjuvant setting. The use of Ki-67 reduction as the primary endpoint is validated in this context, as the magnitude of reduction can be taken as a surrogate for endocrine efficacy. However, the authors explicitly state that the study is small and the results need to be confirmed in a larger number of patients for a longer treatment period before it can be definitively concluded that flaxseed has the potential to reduce the growth and invasiveness of breast cancer. The significant correlations between total flaxseed intake and changes in c-erbB2 and apoptosis index strengthen the biological plausibility of the observed effect.
Applicability
The findings are applicable to postmenopausal patients with newly diagnosed, operable breast cancer. The intervention—25g of ground flaxseed consumed daily in a muffin—is a well-tolerated dietary change. This makes it particularly attractive for consideration in breast cancer prevention or as a potential dietary adjunct to currently used breast cancer drugs, given its excellent tolerability, low cost, and ready availability compared to pharmaceutical options.
Research Objective and Design
Research Objective
The study aimed to determine the effects of daily dietary flaxseed on parameters reflecting tumor growth kinetics and on urinary lignan excretion when given preoperatively to newly diagnosed postmenopausal breast cancer patients.
Study Design
-
Design: Randomized, placebo-controlled, double-blind, prospective study.
-
Allocation: Patients were randomly assigned using a technique of random permutation.
-
Intervention: Patients were randomized to receive a daily muffin containing either:
-
25g of flaxseed (n = 19).
-
Placebo (control) muffin (n = 13). The placebo muffin used whole-wheat flour and added canola oil to be isocaloric and equivalent in fat, protein, and dietary fiber to the flaxseed muffin.
-
-
Duration: The intervention period was between the time of core biopsy diagnosis and definitive surgical excision. Mean treatment times were 32.1 days (range 13-55) for the flaxseed group and 38.7 days (range 16-76) for the placebo group.
-
Outcomes Measured: Ki-67 labeling index (primary end point), apoptosis, c-erbB2 expression, ER and PR receptor levels (analyzed in tumor tissue), and urinary lignan excretion.
Setting and Participants
-
Setting: University Health Network, Toronto.
-
Participants: 32 eligible postmenopausal patients completed the trial.
-
Eligibility Criteria: Postmenopausal for at least 6 months, histologically diagnosed breast carcinoma by core biopsy, no hormone therapy or soy/flaxseed consumption within 90 days of first biopsy, and sufficient tissue specimen for biomarker analysis.
Bibliographic Data
-
Title: Dietary Flaxseed Alters Tumor Biological Markers in Postmenopausal Breast Cancer
-
Authors: Lilian U. Thompson, Jian Min Chen, Tong Li, Kathrin Strasser-Weippl, and Paul E. Goss
-
Journal: Clinical Cancer Research
-
Year: 2005
Mandatory Disclaimer: “This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.”
Original Article:
Full text here
