Comment:
This article was a source of great concern to patients and oncologists when it first came out, and still is. As published in ASCO:
“Using antioxidants and other dietary supplements before and during adjuvant chemotherapy for breast cancer may increase the risk of recurrence and “to a lesser extent, death,” according to an analysis of dietary and nutritional data from a phase III trial, published in the Journal of Clinical Oncology.
With results including a 41% increase in risk of recurrence with supplement use, “you have to conclude that it is probably not beneficial to take supplements if you are otherwise taking in a good diet, and that furthermore, you may cause harm, in the sense that your treatment is going to be less effective,” “
Except, that’s actually not what the study showed, and in fact the opposite. The study, despite bending over backwards to try and find any association, found no statistically significant increase in cancer with supplement use.
Except for B12 and Iron-
The association with B12 and cancer goes back to the 1950s where it was seen that high B12 levels were seen in cancer irrespective of supplements, even theorizing that the cancer cells synthesized high levels of B12.
Iron was also shown to increase
Based on this and previous studies, I’ve removed both B12 and Iron from my multi-vitamin that I give to oncology patients in active cancer treatment.
Summary:
Clinical Bottom Line
This observational study suggests a potential association between the use of certain supplements (notably vitamin B12 and iron) and poorer breast cancer outcomes. However, it critically fails to provide statistically significant evidence for its primary hypothesis: that antioxidant use is harmful. The study’s key finding for “any antioxidant” use (vitamins A, C, E, carotenoids, coenzyme Q10) both before and during chemotherapy had an adjusted hazard ratio (adjHR) for recurrence of 1.41, but the 95% confidence interval crossed 1.0 (0.98 to 2.04), and the P value of .06 did not meet the conventional threshold for statistical significance.
The study’s conclusions are further weakened by questionable methodological choices. Combining chemically diverse compounds into a single “any antioxidant” group is a significant flaw that masks potential differences and assumes they are biologically equivalent. Furthermore, combining “use before and during” chemotherapy into a single exposure category makes it impossible to isolate the effect of supplement use during treatment from the effect of habitual use before treatment.
Results in Context
Main Results
-
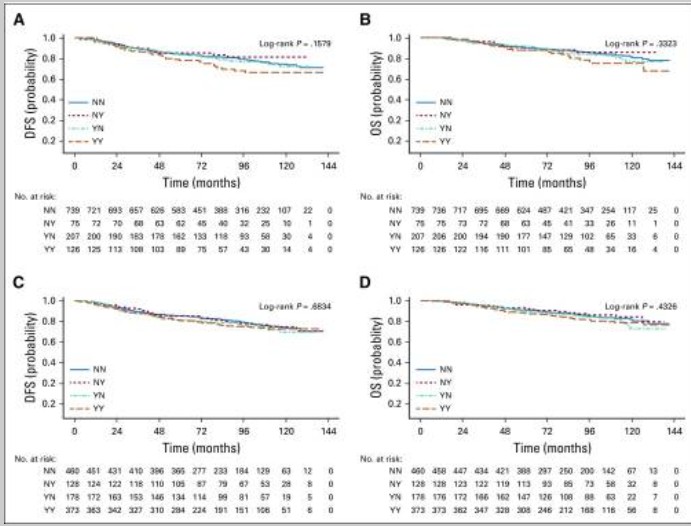
Any Antioxidant Use (Primary Analysis): Use of “any antioxidant” (defined as vitamins A, C, E, carotenoids, or coenzyme Q10) 88both before and during treatment was associated with an increased hazard of recurrence, but this finding was not statistically significant (adjHR, 1.41; 95% CI, 0.98 to 2.04; $P = .06$)99. The association with death was weaker and also not statistically significant (adjHR, 1.40; 95% CI, 0.90 to 2.18; $P = .14$).
-
Individual Antioxidants: Associations for the majority of single antioxidant supplements were also not statistically significant.
-
Statistically Significant Findings:
-
Vitamin B12: Use both before and during chemotherapy was significantly associated with poorer disease-free survival (adjHR, 1.83; 95% CI, 1.15 to 2.92; P < .01) and poorer overall survival (adjHR, 2.04; 95% CI, 1.22 to 3.40; P < .01).
-
Iron: Use during chemotherapy was significantly associated with recurrence (adjHR, 1.79; 95% CI, 1.20 to 2.67; $P < .01$).
-
-
No Association: Multivitamin use was not associated with survival outcomes.
Definitions
A hazard ratio (HR) represents the relative hazard of an event (e.g., recurrence) in the exposed group compared to a reference group. An adjusted HR of 1.41 means there was a 41% increase in the hazard of recurrence in the “any antioxidant” group compared to the non-user group, after adjusting for other clinical and lifestyle variables. The 95% Confidence Interval (CI) is the range of plausible values for the true HR. Because the 95% CI for the main antioxidant finding (0.98 to 2.04) included the value of 1.0 (which signifies no effect), the result is considered statistically non-significant.
Participants
The final analysis included 1,134 patients with high-risk breast cancer who completed questionnaires both before starting chemotherapy (Q1) and 6 months later (Q2).
Assertive Critical Appraisal
Limitations & Bias (STROBE Framework)
-
Lack of Statistical Significance and Power: The study’s primary finding regarding “any antioxidant” use was not statistically significant ($P = .06$). The authors explicitly concede that the “fairly small numbers of patients taking antioxidants… reduced our statistical power”. This resulted in “unstable” risk estimates and is the most likely reason the study failed to demonstrate a statistically robust association for its main hypothesis.
-
Inappropriate Aggregation of Supplements: A major methodological flaw is the decision to combine all antioxidants (vitamins A, C, E, carotenoids, coenzyme Q10) into a single “any antioxidant” variable. This approach is clinically inappropriate as it assumes these chemically distinct compounds have an identical biological mechanism and effect on chemotherapy, which is highly unlikely. This aggregation masks any potential differential effects (beneficial or harmful) of individual supplements and makes the non-significant group finding uninterpretable.
-
Conflated Exposure Timing: The analysis strategy created combined categories, such as “YY” (use both before and during chemotherapy). While this identifies habitual users, it is an inappropriate method for isolating the effect of supplements during chemotherapy. It conflates any potential interaction with chemotherapy with the effects of long-term habitual use, which may be related to underlying conditions (confounding by indication) that themselves are associated with poorer outcomes.
-
Confounding by Indication: As in any observational study, confounding is a critical limitation. Patients who take supplements may be different from those who do not in unmeasured ways. For example, the authors note that habitual use of B12 or iron may be due to a pre-existing condition like anemia, which is itself a known prognostic factor for poorer outcomes. Although the authors adjusted for hematologic toxicity, this does not fully account for the underlying indication for the supplement.
Reporting Quality Assessment (STROBE)
The authors do clearly describe their efforts to address potential sources of confounding . They performed fully adjusted Cox proportional hazards regression models that accounted for dosing arm, age, tumor characteristics (ER, PR, HER2, node status, size), toxicities, BMI, physical activity, smoking, and alcohol consumption .
Applicability
The study population consisted of patients with high-risk breast cancer receiving a specific chemotherapy regimen (doxorubicin, cyclophosphamide, and paclitaxel). The prevalence of supplement use in this trial was noted to be low compared to other reports. The authors suggest this may be due to higher compliance among clinical trial participants , which may limit the generalizability of these findings to a broader clinical practice where supplement use may be more common.
Research Objective
The study’s objective was to prospectively evaluate the associations between the use of dietary supplements, particularly antioxidants, and breast cancer outcomes (disease-free survival and overall survival) among patients enrolled in the SWOG S0221 therapeutic trial.
Study Design
This was a prospective, observational cohort study (named DELCAP) conducted as an ancillary study to a phase III randomized controlled trial (SWOG S0221). Data on supplement use (at least once per week) was collected via patient-reported questionnaires at baseline (Q1, before chemotherapy) and at 6 months (Q2, upon completion of chemotherapy). A 6-month landmark survival analysis was used to assess outcomes.
Setting and Participants
The study included 1,134 patients from institutions participating in the SWOG S0221 trial for high-risk breast cancer. Participants were those who consented to the ancillary DELCAP study and completed both questionnaires.
Key Results for B12 Use
The use of Vitamin B12 was statistically significantly associated with poorer breast cancer outcomes, specifically for patients who reported using it both before and during chemotherapy.
This was one of the strongest associations found in the entire study.
Key Results for Vitamin B12 Use
For the group of patients who used Vitamin B12 both before and during chemotherapy (41 patients, or 3.6% of the study population), the study found:
-
Increased Risk of Recurrence (Disease-Free Survival):
-
Adjusted Hazard Ratio (adjHR): 1.83
-
95% Confidence Interval (CI): 1.15 to 2.92
-
This means this group had an 83% higher hazard of recurrence compared to non-users. The finding is statistically significant because the entire 95% CI is above 1.0.
-
-
Increased Risk of Death (Overall Survival):
-
Adjusted Hazard Ratio (adjHR): 2.04
-
95% Confidence Interval (CI): 1.22 to 3.40
-
This means this group had a 104% higher hazard of death (i.e., more than double the hazard) compared to non-users. This finding is also statistically significant.
-
Use of B12 only before or only during chemotherapy was not associated with statistically significant risk. The finding was specific to the group with habitual use that continued through treatment.
Author Interpretation
The authors noted this was a strong, unexpected finding. They discussed that this could be due to “confounding by indication,” meaning patients might have been taking B12 for pre-existing conditions (like anemia or chemotherapy-induced neuropathy) that are themselves risk factors for poorer outcomes. However, they also noted that B12 is “required for synthesis of DNA in proliferating cells,” suggesting a potential biological mechanism for interacting with chemotherapy.
Key Results for Iron Use
The study found that patients using iron supplements had an increased risk of recurrence (poorer disease-free survival, DFS) and death (poorer overall survival, OS). The results differed slightly based on the timing of use:
-
Use During Chemotherapy Only (NY group):
-
This group had a statistically significant increase in the hazard of recurrence (Adjusted Hazard Ratio [adjHR], 1.79; 95% CI, 1.20 to 2.67; $P < .01$).
-
This group also had a statistically significant increase in the hazard of death (adjHR, 1.62; 95% CI, 1.02 to 2.58).
-
-
Use Both Before and During Chemotherapy (YY group):
-
This group had an increased hazard of recurrence (adjHR, 1.91; 95% CI, 0.98 to 3.70), which was of borderline statistical significance (P = .06).
-
However, this group had a statistically significant increase in the hazard of death (adjHR, 2.67; 95% CI, 1.36 to 5.27).
-
-
Use Before Chemotherapy Only (YN group):
-
This group showed no significant association with recurrence or death.
-
The authors specifically describe these associations as “striking”. In summary, the findings indicate that iron use during chemotherapy, whether started recently or as part of a habitual pattern, was associated with an increased risk of breast cancer recurrence and mortality.
Prevalence of Iron Use
The number of patients taking iron was low. Out of the 1,134 participants:
-
9.6% (109 patients) used iron during treatment.
-
1.9% (22 patients) used iron both before and during treatment.
Author Interpretation and Discussion
The authors discussed several possibilities for this finding:
-
Confounding by Indication: They note that habitual iron use might be due to a pre-existing condition like anemia, which is itself a known risk factor for poorer cancer outcomes.
-
Counter-argument: However, they also point out two things that weaken the “confounding” argument:
-
Adjusting the models for hematologic toxicities did not reduce the observed associations.
-
The association was also seen in patients who only used iron during treatment, not just habitual users.
-
-
Biologic Plausibility: The authors suggest iron supplementation may independently play a role in poorer outcomes. They cite evidence that iron can “play unique roles in tumor initiation and progression” , is required by tumors for proliferation, and may impair antitumor immunity.
Bibliographic Data
-
Title: Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221)
-
Authors: Christine B. Ambrosone, PhD; Gary R. Zirpoli, PhD; Alan D. Hutson, PhD; William E. McCann¹; Susan E. McCann, PhD, RD; et al.
-
Journal: Journal of Clinical Oncology
-
Year: 2019 (published online Dec 19), 2020 (print issue)
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text: Pubmed Central
