Comment:
This article shows an increase in ovarian cancer risk with longer term use of HRT. Both estrogen alone and estrogen-progesterone showed an increase, and the association was stronger in the cohort studies than the case control studies, which are the more robust .
-
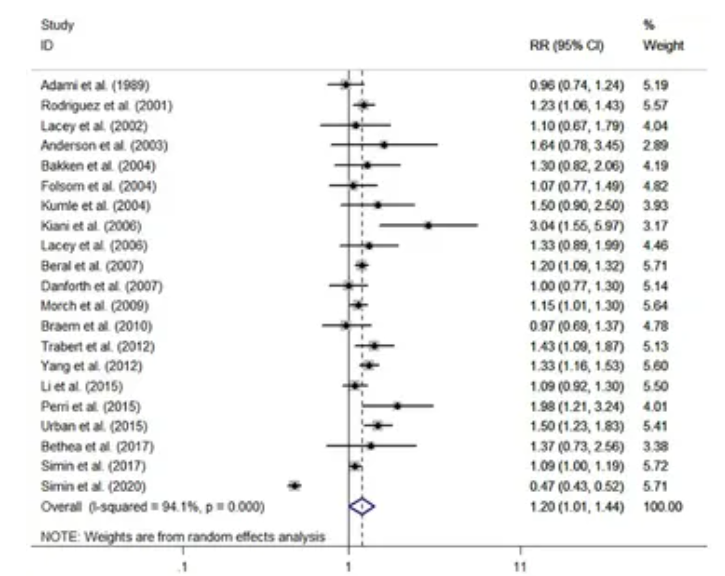
Cohort Studies: The 21 cohort studies showed a slightly higher risk. The pooled relative risk (RR) was 1.20, with a 95% confidence interval of 1.01 to 1.44. This means HRT users had a 20% increased risk of developing ovarian cancer compared to non-users. However, the confidence interval is quite wide and the lower end is very close to 1.0, which indicates a lower level of certainty in this finding.
-
Case-Control Studies: The 30 case-control studies found a slightly lower risk. The pooled odds ratio (OR) was 1.13, with a 95% confidence interval of 1.04 to 1.22. This represents a 13% increase in the odds of having ovarian cancer among HRT users. The confidence interval is narrower, suggesting a more statistically precise result, even if the effect size is smaller.
Summary:
Clinical Bottom Line
This meta-analysis of observational studies finds a small but statistically significant increase in the risk of ovarian cancer among users of hormone replacement therapy (HRT). However, this elevated risk appears to be a historical finding; when the analysis was restricted to studies published in the last 15-20 years, the association was no longer statistically significant, suggesting that modern HRT regimens may be safer. The risk is most pronounced with long-term use, particularly for more than 10 years, and appears higher for serous and endometrioid ovarian cancers. While this review provides reassurance about the risk profile of modern, short-term HRT, the data still support a cautious approach, especially for long-duration therapy.
Results
Summary of Results
The meta-analysis pooled data from 51 observational studies, analyzing cohort and case-control studies separately.
-
Overall Risk:
-
From 21 cohort studies, HRT users had a pooled relative risk (RR) of ovarian cancer of 1.20 (95% CI, 1.01-1.44) compared to non-users.
-
From 30 case-control studies, the pooled odds ratio (OR) was 1.13 (95% CI, 1.04-1.22).
-
-
Risk in Recent Studies: When the analysis was limited to more recent publications, the increased risk was no longer statistically significant.
-
Cohort studies after 2010: Pooled RR was 1.15 (95% CI, 0.82-1.61).
-
Case-control studies after 2006: Pooled OR was 1.09 (95% CI, 0.93-1.27).
-
-
Risk by Duration of Use (from Cohort Studies): The risk increased with the length of HRT use.
-
< 5 years: RR 1.07 (95% CI, 0.96-1.19).
-
5-9 years: RR 1.39 (95% CI, 1.20-1.62).
-
≥ 10 years: RR 1.52 (95% CI, 1.31-1.77).
-
-
Risk by Histologic Subtype (from Cohort Studies): HRT was associated with an increased risk of specific cancer types.
-
Serous cancer: RR 1.57 (95% CI, 1.43-1.72).
-
Endometrioid cancer: RR 1.44 (95% CI, 1.00-2.06).
-
Assertive Critical Appraisal
Certainty of Evidence (GRADE Framework)
The authors did not use the GRADE framework to rate the certainty of evidence. Based on this appraisal, the overall certainty of the evidence is low to moderate. This is because the findings are based exclusively on observational studies, which cannot prove causation, and the analyses showed significant heterogeneity, indicating inconsistency across the included studies.
Heterogeneity
The analysis found considerable statistical heterogeneity, which is the percentage of variation across studies due to real differences rather than just chance.
-
The cohort studies showed high heterogeneity (I2=94.1%). This level of inconsistency is very high and suggests that the pooled average risk should be interpreted with caution, as the results of the individual studies differ substantially.
-
The case-control studies showed moderate heterogeneity (I2=58.6%).
A meta-regression analysis failed to identify a specific reason for this high level of variability.
Publication Bias
The authors assessed for publication bias—the possibility that studies with negative results are less likely to be published—and reported no significant bias was detected using Begg’s and Egger’s tests. However, the p-value for the Egger’s test (P=0.070) was close to the authors’ chosen threshold for significance (P<0.10), suggesting a potential for bias that cannot be entirely ruled out.
Risk of Bias in Included Studies
The authors report that the included studies received a “moderate or high-quality score” based on the Newcastle-Ottawa Quality Assessment Form. While reassuring, this is a summary statement, and pooling data from numerous observational studies, even those of moderate quality, can compound underlying biases related to confounding and measurement.
Reporting Quality Assessment (PRISMA)
The review follows key PRISMA reporting guidelines, including the provision of a PRISMA flow diagram showing the study selection process. However, the paper does not report the full search strategy for at least one database, which is an omission that reduces the transparency and reproducibility of the systematic review.
Research Objective
The stated objective was to assess the risk of ovarian cancer associated with HRT, considering factors such as the time period of the study, duration of HRT use, type of hormone regimen, and specific histologic subtypes of ovarian cancer.
Study Design
This was a systematic review and meta-analysis of published observational studies26. The authors searched five major databases (PUBMED, OVID, Embase, Cochrane, and Web of Science) for cohort and case-control studies published between 1980 and April 202227272727. The analysis included studies that reported a relative risk (RR) or odds ratio (OR) for pathologically confirmed ovarian cancer in HRT users versus non-users.
Setting and Participants
The analysis included a total of 51 studies
-
21 Cohort Studies: Involving 15,313 ovarian cancer cases among 4,564,785 participants
-
30 Case-Control Studies: Including 18,738 ovarian cancer cases and 57,747 controls
Bibliographic Data
-
Title: The risk of ovarian cancer in hormone replacement therapy users: a systematic review and meta-analysis
-
Authors: Xiang H, Wang L, Sun L and Xu S
-
Journal: Frontiers in Endocrinology
-
Year: 2024
-
DOI: 10.3389/fendo.2024.1414968
This AI-generated analysis is for informational and research purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Original Article:
Full text pdf: The risk of ovarian cancer in hormone replacement therapy users- a systematic review and meta-analysis
Open Access Copyright © 2024 Xiang, Wang, Sun and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
