Comment:
Metronomic chemotherapy (mCHT) is a concept that’s been around, but it’s often overlooked in favor of high-dose regimens. This 2022 review from J. Clin. Med. brilliantly frames the evidence, separating the clear “lights”—like its low-toxicity profile—from the persistent “shadows” we still face, such as the lack of large randomized trials. For our frail or heavily pre-treated patients with advanced breast cancer, this approach offers a crucial, quality-of-life-preserving alternative.
Summary:
💡 Clinical Bottom Line
Metronomic chemotherapy (mCHT) is a suitable and well-tolerated treatment option for selected patients with advanced breast cancer (ABC), with its most significant “light” being its remarkably low toxicity profile. In combination with targeted agents, mCHT offers a therapeutic alternative, especially for frail or elderly patients, due to the ease of oral administration and reduced side effects compared to standard maximum tolerated dose (MTD) chemotherapy.
However, the field is hampered by significant “shadows,” including a lack of prospective randomized trials comparing mCHT to standard CHT, heterogeneity in dosing/schedules, and an absence of validated predictive biomarkers. Clinicians must remain aware that mCHT’s low toxicity advantage is partially offset when combined with targeted agents that introduce their own toxicities.
🔆 Lights (Established Findings/Advantages)
-
Low Toxicity Profile (Clinical): This is considered the brightest light. Across multiple studies, mCHT (especially regimens of cyclophosphamide, methotrexate, vinorelbine, and capecitabine) is associated with low rates of severe (Grade 3/4) adverse events.
-
Quality of Life (QoL) Preservation (Clinical): Studies suggest mCHT can maintain or even improve QoL compared to standard regimens, with patients reporting substantially less hair loss and less numbness/tingling.
-
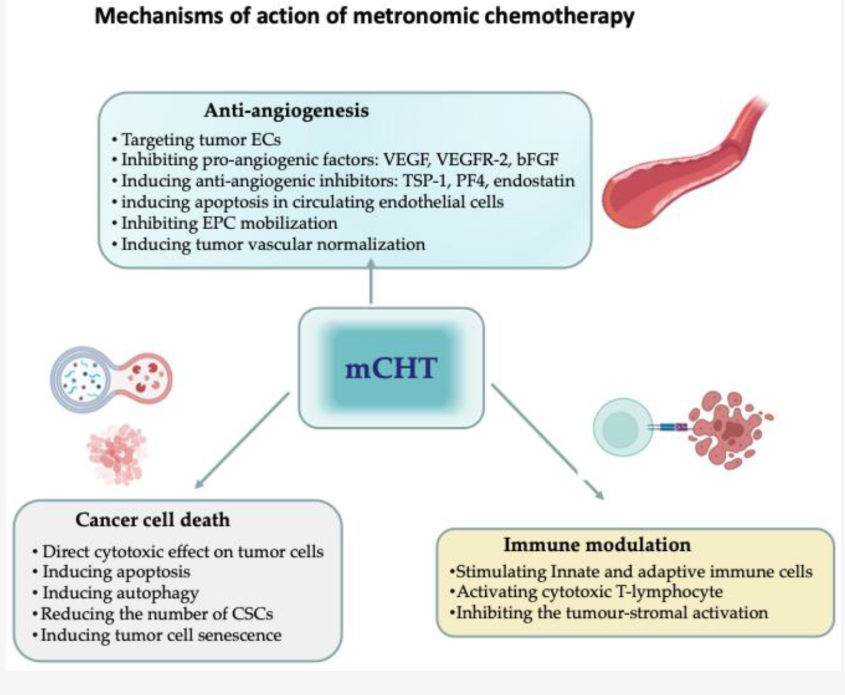
Anti-Angiogenesis (Preclinical): A well-documented mechanism is the prevention of tumor angiogenesis. mCHT preferentially targets proliferating endothelial cells, inhibits pro-angiogenic factors (e.g., VEGF, VEGFR-2), and induces anti-angiogenic inhibitors (e.g., TSP-1).
-
Immune Modulation (Preclinical): mCHT stimulates the innate and adaptive immune systems, which can be leveraged in combination with immune checkpoint inhibitors.
🌑 Shadows (Challenges/Areas Needing Research)
-
Lack of Randomized Trials (Clinical): A crucial limitation is the almost complete absence of large, prospective randomized trials to directly compare mCHT with standard-dosing regimens in advanced breast cancer (ABC).
-
Optimal Biological Dose (OBD) and Scheduling (Clinical/Preclinical): There is a lack of definition for an optimal biological dose (OBD)—the smallest effective dose with minimal toxicity—and a vast heterogeneity in administration schedules.
-
Biomarker Validation (Clinical/Preclinical): Solid and reliable predictive biomarkers are still needed to select which patients will most benefit from mCHT.
-
Uncertainty of Direct Tumor Cell Effects (Preclinical): The direct cytotoxic effects on tumor cells are less well-defined compared to the anti-angiogenic and immunomodulatory effects.
🔎 Assertive Critical Appraisal
Study Design and Evidence Quality
-
Source of Bias: The main weakness of the field is the heavy reliance on small, mostly retrospective, single-arm studies and a variety of different regimens and schedules. This high level of heterogeneity makes definitive conclusions about efficacy difficult to establish.
-
Biomarker Methodological Flaw: The review highlights the major gap between preclinical data on the mechanism of action (e.g., anti-angiogenesis) and the prospective collection of corresponding circulating biomarkers in clinical trials.
-
Quality of Life (PROs) Appraisal: The lack of routine, prospective QoL assessment in mCHT trials is a major omission.
Applicability
The findings are highly applicable to general clinical practice, particularly for the management of advanced breast cancer patients who are frail, elderly, or heavily pre-treated, where avoiding cumulative toxicity and preserving quality of life are paramount goals.
📋 Research Summary
-
Research Objective: The present review aims to identify and discuss the “lights and shadows” of metronomic chemotherapy (mCHT) in both preclinical and clinical settings for the treatment of metastatic breast cancer.1
-
Type: Narrative and critical review of the literature.
-
Inclusion Criteria (Clinical): Focus on studies reporting side-effects, quality of life (QoL), or symptom control, specifically including only pure metronomic regimens for toxicity analysis.
📚 Bibliographic Data
Title: Metronomic Chemotherapy for Metastatic Breast Cancer Treatment: Clinical and Preclinical Data between Lights and Shadows
Authors: Marina Elena Cazzaniga, Serena Capici, Nicoletta Cordani, Viola Cogliati, Francesca Fulvia Pepe, Francesca Riva and Maria Grazia Cerrito
Journal: J. Clin. Med.
Year: 2022
Original Article:
Full text pdf: Metronomic Chemotherapy for Metastatic Breast Cancer Treatment Clinical and Preclinical Data between Lights and Shadows
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
